Phylogenetically Defined Isoforms of Listeria monocytogenes Invasion Factor InlB Differently Activate Intracellular Signaling Pathways and Interact with the Receptor gC1q-R
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Properties of Idinlb Isoforms
2.2. Kinetics of Activation of MAPK and PI3K Signaling Pathways by idInlBs was Different foridInlB Isoforms
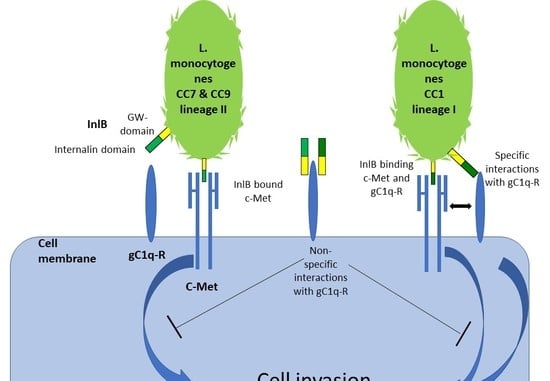
2.3. idInlBs Isoforms Interacted with gCq1-R In Vitro in a Lineage-Specific Manner
2.4. gC1q-R Antibodies Specifically Inhibited Lineage I idInlB- but not Lineage II idInlB-Driven Invasion into HEp-2 Cells
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Protein Purification
4.3. Cell Culture and Growth Conditions
4.4. Size Exclusion Chromatography (SEC)
4.5. Fluorescence Spectra
4.6. Immunoblotting
4.7. Solid-Phase Microplate Binding and Competition Assay
4.8. Invasion Assay
4.9. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CC | Clonal complex |
| id | Internalin domain |
| idInlB | InlB internalin domain |
| SEC | Size-exclusive chromatography |
| ST | Sequence type |
References
- De Noordhout, C.M.; Devleesschauwer, B.; Angulo, F.J.; Verbeke, G.; Haagsma, J.; Kirk, M.; Havelaar, A.; Speybroeck, N. The global burden of listeriosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 1073–1082. [Google Scholar] [CrossRef]
- Vázquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Domínguez-Bernal, G.; Goebel, W.; González-Zorn, B.; Wehland, J.; Kreft, J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef] [PubMed]
- Allerberger, F.; Wagner, M. Listeriosis: A resurgent foodborne infection. Clin. Microbiol. Infect. 2010, 16, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Perrodeau, É.; Leclercq, A.; Cazenave, B.; Pilmis, B.; Henry, B.; Lopes, A.; Maury, M.M.; Moura, A.; Goffinet, F.; et al. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect. Dis. 2017, 17, 510–519. [Google Scholar] [CrossRef]
- Mylonakis, E.; Paliou, M.; Hohmann, E.L.; Calderwood, S.B.; Wing, E.J. Listeriosis during pregnancy: A case series and review of 222 cases. Medicine (Baltimore) 2002, 81, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Boland, J.A.; Krypotou, E.; Scortti, M. Listeria Placental Infection. MBio 2017, 8, e00949-17. [Google Scholar] [CrossRef] [PubMed]
- Girard, D.; Leclercq, A.; Laurent, E.; Lecuit, M.; De Valk, H.; Goulet, V. Pregnancy-related listeriosis in France, 1984 to 2011, With a focus on 606 cases from 1999 to 2011. Eurosurveillance 2014, 19, 20909. [Google Scholar] [CrossRef] [PubMed]
- Pizarro-Cerdá, J.; Kühbacher, A.; Cossart, P. Entry of listeria monocytogenes in mammalian epithelial cells: An updated view. Cold Spring Harb. Perspect. Med. 2012, 2, a010009. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Cossart, P. Microbe Profile: Listeria monocytogenes: a paradigm among intracellular bacterial pathogens. Microbiology 2019, 165, 719–721. [Google Scholar] [CrossRef]
- Lecuit, M. Understanding how Listeria monocytogenes targets and crosses host barriers. Clin. Microbiol. Infect. 2005, 11, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Nightingale, K.K.; Windham, K.; Wiedmann, M. Evolution and molecular phylogeny of Listeria monocytogenes isolated from human and animal listeriosis cases and foods. J. Bacteriol. 2005, 187, 5537–5551. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, O.F.; Skouboe, P.; Dons, L.; Rosen, L.; Olsen, J.E. Listeria monocytogenes exists in at least three evolutionary lines: Evidence from flagellin, invasive associated protein and listeriolysin O genes. Microbiology 1995, 141, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.H.; de Bakker, H.C.; Wiedmann, M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Zadoks, R.N.; Fortes, E.D.; Dogan, B.; Cai, S.; Chen, Y.; Scott, V.N.; Gombas, D.E.; Boor, K.J.; Wiedmann, M. Listeria monocytogenes isolates from foods and humans form distinct but overlapping populations. Appl. Environ. Microbiol. 2004, 70, 5833–5841. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Doumith, M.; Duperrier, S.; Giovannacci, I.; Morvan, A.; Glaser, P.; Buchrieser, C.; Jacquet, C.; Martin, P. Genetic diversity of Listeria monocytogenes recovered from infected persons and pork, seafood and dairy products on retail sale in France during 2000 and 2001. Int. J. Food Microbiol. 2007, 114, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, M.; Aguilar-Bultet, L.; Rupp, S.; Guldimann, C.; Stephan, R.; Schock, A.; Otter, A.; Schüpbach, G.; Brisse, S.; Lecuit, M.; et al. Listeria monocytogenes sequence type 1 is predominant in ruminant rhombencephalitis. Sci. Rep. 2016, 6, 36419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennison, A.V.; Masson, J.J.; Fang, N.X.; Graham, R.M.; Bradbury, M.I.; Fegan, N.; Gobius, K.S.; Graham, T.M.; Guglielmino, C.J.; Brown, J.L.; et al. Analysis of the Listeria monocytogenes population structure among isolates from 1931 to 2015 in Australia. Front. Microbiol. 2017, 8, 603. [Google Scholar] [CrossRef]
- Maury, M.M.; Tsai, Y.H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2017, 49, 970. [Google Scholar] [CrossRef]
- Hilliard, A.; Leong, D.; O’Callaghan, A.; Culligan, E.P.; Morgan, C.A.; Delappe, N.; Hill, C.; Jordan, K.; Cormican, M.; Gahan, C.G.M. Genomic characterization of listeria monocytogenes isolates associated with clinical listeriosis and the food production environment in Ireland. Genes (Basel) 2018, 9, 171. [Google Scholar] [CrossRef]
- Liu, D.; Lawrence, M.L.; Gorski, L.; Mandrell, R.E.; Ainsworth, A.J.; Austin, F.W. Listeria monocytogenes serotype 4b strains belonging to lineages I and III possess distinct molecular features. J. Clin. Microbiol. 2006, 44, 214–217. [Google Scholar] [CrossRef]
- Roberts, A.; Nightingale, K.; Jeffers, G.; Fortes, E.; Kongo, J.M.; Wiedmann, M. Genetic and phenotypic characterization of Listeria monocytogenes lineage III. Microbiology 2006, 152, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.H.L.; Maron, S.B.; McGann, P.; Nightingale, K.K.; Wiedmann, M.; Orsi, R.H. Recombination and positive selection contributed to the evolution of Listeria monocytogenes lineages III and IV, two distinct and well supported uncommon L. monocytogenes lineages. Infect. Genet. Evol. 2011, 11, 1881–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, A.; Criscuolo, A.; Pouseele, H.; Maury, M.M.; Leclercq, A.; Tarr, C.; Björkman, J.T.; Dallman, T.; Reimer, A.; Enouf, V.; et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2016, 2, 16185. [Google Scholar] [CrossRef] [PubMed]
- Kuenne, C.; Billion, A.; Mraheil, M.A.; Strittmatter, A.; Daniel, R.; Goesmann, A.; Barbuddhe, S.; Hain, T.; Chakraborty, T. Reassessment of the Listeria monocytogenes pan-genome reveals dynamic integration hotspots and mobile genetic elements as major components of the accessory genome. BMC Genomics 2013, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Maury, M.M.; Chenal-Francisque, V.; Bracq-Dieye, H.; Han, L.; Leclercq, A.; Vales, G.; Moura, A.; Gouin, E.; Scortti, M.; Disson, O.; et al. Spontaneous loss of virulence in natural populations of Listeria monocytogenes. Infect. Immun. 2017, 85, e00541-17. [Google Scholar] [CrossRef] [PubMed]
- Quereda, J.J.; Dussurget, O.; Nahori, M.-A.; Ghozlane, A.; Volant, S.; Dillies, M.-A.; Regnault, B.; Kennedy, S.; Mondot, S.; Villoing, B.; et al. Bacteriocin from epidemic Listeria strains alters the host intestinal microbiota to favor infection. Proc. Natl. Acad. Sci. USA 2016, 113, 5706–5711. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Nagai, T.; Hayashi, T.; Baba, Y.; Nagai, S.; Koyasu, S. Listerial invasion protein internalin B promotes entry into ileal Peyer’s patches in vivo. Microbiol. Immunol. 2011, 55, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, C.; Doumith, M.; Gordon, J.I.; Martin, P.M.V.; Cossart, P.; Lecuit, M. A Molecular Marker for Evaluating the Pathogenic Potential of Foodborne Listeria monocytogenes. J. Infect. Dis. 2004, 189, 2094–2100. [Google Scholar] [CrossRef]
- Nightingale, K.K.; Ivy, R.A.; Ho, A.J.; Fortes, E.D.; Njaa, B.L.; Peters, R.M.; Wiedmann, M. inlA premature stop codons are common among Listeria monocytogenes isolates from foods and yield virulence-attenuated strains that confer protection against fully virulent strains. Appl. Environ. Microbiol. 2008, 74, 6570–6583. [Google Scholar] [CrossRef]
- Ferreira da Silva, M.; Ferreira, V.; Magalhães, R.; Almeida, G.; Alves, A.; Teixeira, P. Detection of premature stop codons leading to truncated internalin A among food and clinical strains of Listeria monocytogenes. Food Microbiol. 2017, 63, 6–11. [Google Scholar] [CrossRef]
- Bierne, H.; Cossart, P. InlB, a surface protein of Listeria monocytogenes that behaves as an invasin and a growth factor. J. Cell Sci. 2002, 115, 3357–3367. [Google Scholar] [PubMed]
- Tsai, Y.H.L.; Orsi, R.H.; Nightingale, K.K.; Wiedmann, M. Listeria monocytogenes internalins are highly diverse and evolved by recombination and positive selection. Infect. Genet. Evol. 2006, 6, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Adgamov, R.; Zaytseva, E.; Thiberge, J.-M.; Brisse, S.; Ermolaeva, S. Genetically Related Listeria Monocytogenes Strains Isolated from Lethal Human Cases and Wild Animals; Caliskan, M., Ed.; InTech Open: London, UK, 2012. [Google Scholar]
- Voronina, O.L.; Ryzhova, N.N.; Kunda, M.S.; Kurnaeva, M.A.; Semenov, A.N.; Aksenova, E.I.; Egorova, I.Y.; Kolbasov, D.V.; Ermolaeva, S.A.; Gintsburg, A.L. Diversity and Pathogenic Potential of Listeria monocytogenes Isolated from Environmental Sources in the Russian Federation. Int. J. Mod. Eng. Res. 2015, 5, 5–15. [Google Scholar]
- Sobyanin, K.; Sysolyatina, E.; Krivozubov, M.; Chalenko, Y.; Karyagina, A.; Ermolaeva, S. Naturally occurring InlB variants that support intragastric Listeria monocytogenes infection in mice. FEMS Microbiol. Lett. 2017, 364, fnx011. [Google Scholar] [CrossRef] [PubMed]
- Sobyanin, K.A.; Sysolyatina, E.V.; Chalenko, Y.M.; Kalinin, E.V.; Ermolaeva, S.A. Route of Injection Affects the Impact of InlB Internalin Domain Variants on Severity of Listeria monocytogenes Infection in Mice. Biomed. Res. Int. 2017, 2017, 2101575. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Naujokas, M.; Park, M.; Ireton, K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 2000, 103, 501–510. [Google Scholar] [CrossRef]
- Li, N.; Xiang, G.S.; Dokainish, H.; Ireton, K.; Elferink, L.A. The Listeria protein internalin B mimics hepatocyte growth factor-induced receptor trafficking. Traffic 2005, 6, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Gessain, G.; Disson, O.; Lecuit, M. PI3-kinase activation is critical for host barrier permissiveness to Listeria monocytogenes. Med. Sci. (Paris) 2016, 212, 165–183. [Google Scholar] [CrossRef]
- Banerjee, M.; Copp, J.; Vuga, D.; Marino, M.; Chapman, T.; Van Der Geer, P.; Ghosh, P. GW domains of the Listena monocytogenes invasion protein InlB are required for potentiation of Met activation. Mol. Microbiol. 2004, 52, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Braun, L. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 2000, 19, 1458–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copp, J.; Marino, M.; Banerjee, M.; Ghosh, P.; Van der Geer, P. Multiple regions of internalin B contribute to its ability to turn on the Ras-mitogen-activated protein kinase pathway. J. Biol. Chem. 2003, 278, 7783–7789. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, A.; Machner, M.P.; Pfeil, W.; Schubert, W.D.; Heinz, D.W.; Seckler, R. Folding and stability of the leucine-rich repeat domain of internalin B from Listeria monocytogenes. J. Mol. Biol. 2004, 337, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, D.M.; Gherardi, E.; Di, Y.; Heinz, D.W.; Niemann, H.H. Ligand-Mediated Dimerization of the Met Receptor Tyrosine Kinase by the Bacterial Invasion Protein InlB. J. Mol. Biol. 2010, 395, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.H.; Jäger, V.; Butler, P.J.G.; van den Heuvel, J.; Schmidt, S.; Ferraris, D.; Gherardi, E.; Heinz, D.W. Structure of the Human Receptor Tyrosine Kinase Met in Complex with the Listeria Invasion Protein InlB. Cell 2007, 130, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonquières, R.; Pizarro-Cerdá, J.; Cossart, P. Synergy between the N- and C-terminal domains of InlB for efficient invasion of non-phagocytic cells by Listeria monocytogenes. Mol. Microbiol. 2001, 42, 955–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, M.; Banerjee, M.; Jonquières, R.; Cossart, P.; Ghosh, P. GW domains of the Listeria monocytogenes invasion protein InlB are SH3-like and mediate binding to host ligands. EMBO J. 2002, 21, 5623–5634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghebrehiwet, B.; Peerschke, E.I.B. cC1q-R (calreticulin) and gC1q-R/p33: Ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Proc. Mol. Immunol. 2004, 41, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.K.; Hafiz, A.; Banerjee, B.; Kim, K.S.; Datta, K.; Chitnis, C.E. Plasmodium falciparum uses gC1qR/HABP1/p32 as a receptor to bind to vascular endothelium and for platelet-mediated clumping. PLoS Pathog. 2007, 3, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Ghebrehiwet, B.; Tantral, L.; Titmus, M.A.; Panessa-Warren, B.J.; Tortora, G.T.; Wong, S.S.; Warren, J.B. The exosporium of B. cereus contains a binding site for gC1qR/p33: Implication in spore attachment and/or entry. Adv. Exp. Med. Biol. 2007, 598, 181–197. [Google Scholar]
- Choi, Y.; Kwon, Y.C.; Kim, S.I.; Park, J.M.; Lee, K.H.; Ahn, B.Y. A hantavirus causing hemorrhagic fever with renal syndrome requires gC1qR/p32 for efficient cell binding and infection. Virology 2008, 381, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Ragon, M.; Wirth, T.; Hollandt, F.; Lavenir, R.; Lecuit, M.; Le Monnier, A.; Brisse, S. A new perspective on Listeria monocytogenes evolution. PLoS Pathog. 2008, 4, e1000146. [Google Scholar] [CrossRef] [PubMed]
- Glaser, P.; Frangeul, L.; Buchrieser, C.; Rusniok, C.; Amend, A.; Baquero, F.; Berche, P.; Bloecker, H.; Brandt, P.; Chakraborty, T.; et al. Comparative genomics of Listeria species. Science 2001, 294, 849–852. [Google Scholar] [PubMed]
- Zaytseva, E.; Ermolaeva, S.; Somov, G.P. Low genetic diversity and epidemiological significance of Listeria monocytogenes isolated from wild animals in the far east of Russia. Infect. Genet. Evol. 2007, 7, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Témoin, S.; Roche, S.M.; Grépinet, O.; Fardini, Y.; Velge, P. Multiple point mutations in virulence genes explain the low virulence of Listeria monocytogenes field strains. Microbiology 2008, 154, 939–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauerfeind, R.; Barth, S.; Weiß, R.; Baljer, G. Sequence polymorphism of the Salmonella plasmid virulence factor D (SpvD) in Salmonella enterica isolates of animal origin. In Proceedings of the 4th International Symposium on the Epidemiology and Control of Salmonella and other Food Borne Pathogens in Pork; Iowa State University Digital Press : Iowa City, Iowa, 2001; Volume 157, pp. 604–613. [Google Scholar]
- Grabe, G.J.; Zhang, Y.; Przydacz, M.; Rolhion, N.; Yang, Y.; Pruneda, J.N.; Komander, D.; Holden, D.W.; Hare, S.A. The Salmonella effector SpvD is a cysteine hydrolase with a serovar-specific polymorphism influencing catalytic activity, suppression of immune responses, and bacterial virulence. J. Biol. Chem. 2016, 291, 25853–25863. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, F.P.; Guex, N.; Word, J.M.; Miller, L.A.; Becker, J.A.; Walsh, S.L.; Scangarella, N.E.; West, J.M.; Shawar, R.M.; Amrine-Madsen, H. A Geographic Variant of the Staphylococcus aureus Panton-Valentine Leukocidin Toxin and the Origin of Community-Associated Methicillin-Resistant S. aureus USA300. J. Infect. Dis. 2008, 197, 187–194. [Google Scholar] [CrossRef]
- Dumitrescu, O.; Tristan, A.; Meugnier, H.; Bes, M.; Gouy, M.; Etienne, J.; Lina, G.; Vandenesch, F. Polymorphism of the Staphylococcus aureus Panton-Valentine Leukocidin Genes and Its Possible Link with the Fitness of Community-Associated Methicillin-Resistant S. aureus. J. Infect. Dis. 2008, 198, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Han, Y.; Song, Y.; Huang, P.; Yang, R. Comparative and evolutionary genomics of Yersinia pestis. Microbes Infect. 2004, 6, 1226–1234. [Google Scholar] [CrossRef]
- Dentovskaya, S.V.; Platonov, M.E.; Svetoch, T.E.; Kopylov, P.K.; Kombarova, T.I.; Ivanov, S.A.; Shaikhutdinova, R.Z.; Kolombet, L.V.; Chauhan, S.; Ablamunits, V.G.; et al. Two isoforms of Yersinia pestis plasminogen activator Pla: Intraspecies distribution, intrinsic disorder propensity, and contribution to virulence. PLoS ONE 2016, 11, e0168089. [Google Scholar] [CrossRef]
- Haiko, J.; Kukkonen, M.; Ravantti, J.J.; Westerlund-Wikström, B.; Korhonen, T.K. The single substitution I259T, conserved in the plasminogen activator Pla of pandemic Yersinia pestis branches, enhances fibrinolytic activity. J. Bacteriol. 2009, 191, 4758–4766. [Google Scholar] [CrossRef]
- Pednekar, L.; Pathan, A.A.; Paudyal, B.; Tsolaki, A.G.; Kaur, A.; Abozaid, S.M.; Kouser, L.; Khan, H.A.; Peerschke, E.I.; Shamji, M.H.; et al. Analysis of the Interaction between globular head modules of human C1q and its candidate receptor gC1qR. Front. Immunol. 2016, 7, 567. [Google Scholar] [CrossRef]
- Kim, K.B.; Yi, J.S.; Nguyen, N.; Lee, J.H.; Kwon, Y.C.; Ahn, B.Y.; Cho, H.; Kim, Y.K.; Yoo, H.J.; Lee, J.S.; et al. Cell-surface receptor for complement component C1q (gC1qR) is a key regulator for lamellipodia formation and cancer metastasis. J. Biol. Chem. 2011, 286, 23093–23101. [Google Scholar] [CrossRef]
- Chalenko, Y.M.; Sysolyatina, E.V.; Kalinin, E.V.; Sobyanin, K.A.; Ermolaeva, S.A. Natural variants of Listeria monocytogenes internalin B with different ability to stimulate cell proliferation and cytoskeleton rearrangement in HEp-2 cells. Mol. Genet. Microbiol. Virol. 2017, 32, 80–86. [Google Scholar] [CrossRef]
- Pentecost, M.; Kumaran, J.; Ghosh, P.; Amieva, M.R. Listeria monocytogenes internalin B activates junctional endocytosis to accelerate intestinal invasion. PLoS Pathog. 2010, 6, e1000900. [Google Scholar] [CrossRef]
- O’Sullivan, D.J.; Klaenhammer, T.R. High- and low-copy-number Lactococcus shuttle cloning vectors with features for clone screening. Gene 1993, 137, 227–231. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalenko, Y.; Kalinin, E.; Marchenkov, V.; Sysolyatina, E.; Surin, A.; Sobyanin, K.; Ermolaeva, S. Phylogenetically Defined Isoforms of Listeria monocytogenes Invasion Factor InlB Differently Activate Intracellular Signaling Pathways and Interact with the Receptor gC1q-R. Int. J. Mol. Sci. 2019, 20, 4138. https://doi.org/10.3390/ijms20174138
Chalenko Y, Kalinin E, Marchenkov V, Sysolyatina E, Surin A, Sobyanin K, Ermolaeva S. Phylogenetically Defined Isoforms of Listeria monocytogenes Invasion Factor InlB Differently Activate Intracellular Signaling Pathways and Interact with the Receptor gC1q-R. International Journal of Molecular Sciences. 2019; 20(17):4138. https://doi.org/10.3390/ijms20174138
Chicago/Turabian StyleChalenko, Yaroslava, Egor Kalinin, Victor Marchenkov, Elena Sysolyatina, Alexey Surin, Konstantin Sobyanin, and Svetlana Ermolaeva. 2019. "Phylogenetically Defined Isoforms of Listeria monocytogenes Invasion Factor InlB Differently Activate Intracellular Signaling Pathways and Interact with the Receptor gC1q-R" International Journal of Molecular Sciences 20, no. 17: 4138. https://doi.org/10.3390/ijms20174138
APA StyleChalenko, Y., Kalinin, E., Marchenkov, V., Sysolyatina, E., Surin, A., Sobyanin, K., & Ermolaeva, S. (2019). Phylogenetically Defined Isoforms of Listeria monocytogenes Invasion Factor InlB Differently Activate Intracellular Signaling Pathways and Interact with the Receptor gC1q-R. International Journal of Molecular Sciences, 20(17), 4138. https://doi.org/10.3390/ijms20174138