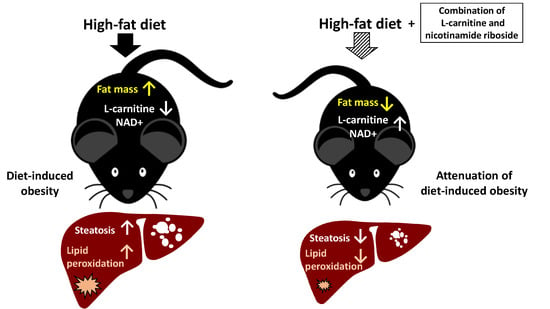
Combined Treatment with L-Carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis
Abstract
:1. Introduction
2. Results
2.1. Experimental Diets Induce Carnitine and Nicotinamide Plasma Levels
2.2. Combined Treatment with L-Carnitine and Nicotinamide Riboside Attenuates HFD-Induced Obesity Independent of Food Intake or Activity
2.3. COMBI Treatment Attenuates Metabolic Risk Factors and Liver Integrity Marker ALT
2.4. COMBI Treatment Reduces HFD-Induced Liver Steatosis
2.5. NR and COMBI Attenuate Hepatic Lipid Peroxidation
2.6. COMBI Counteracts Effects of HFD on Metabolic Pathways in the Liver
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Food Intake, Body Composition
4.3. Plasma Measurements
4.4. Sacrifice and Analysis of Fat and Liver Tissue
4.5. 4-HNE Oxidative Stress-Related Marker Staining and Quantification in Liver Tissue
4.6. LC-MS/MS
4.7. Gene Expression and Pathway Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NAFLD | Non-alcoholic fatty liver disease |
| LC | L-Carnitine |
| NAD+ | Nicotinamide adenine dinucleotide |
| NR | Nicotinamide riboside |
| FA | Fatty acid |
| 4-HNE | 4-Hydroxynonenal |
| COMBI | Combination of LC and NR treatment in this study |
| Ldlr-/- | Low-density lipoprotein receptor knockout |
| HFD | High-fat diet |
| BW | Body weight |
| FI | Food intake |
| WAT | White adipose tissue |
| HDL | High-density lipoprotein |
| ALT | Alanine aminotransferase |
| DEG | Differentially expressed genes |
| RU | Relative units |
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 6736, 1–16. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016. [Google Scholar] [CrossRef]
- Fildes, A.; Charlton, J.; Rudisill, C.; Littlejohns, P.; Prevost, A.T.; Gulliford, M.C. Probability of an obese person attaining normal body weight: Cohort study using electronic health records. Am. J. Public Health 2015. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, E.Y.; Choi, M.S. Dietary isoliquiritigenin at a low dose ameliorates insulin resistance and NAFLD in diet-induced obesity in C57BL/6J mice. Int. J. Mol. Sci. 2018, 19, 3281. [Google Scholar] [CrossRef]
- Schrader, M.; Costello, J.; Godinho, L.F.; Islinger, M. Peroxisome-mitochondria interplay and disease. J. Inherit. Metab. Dis. 2015. [Google Scholar] [CrossRef]
- Wijburg, F.A.; Wüst, R.C.I.; Visser, G.; Wanders, R.J.A.; Knottnerus, S.J.G.; IJlst, L.; Houtkooper, R.H.; Ferdinandusse, S.; Bleeker, J.C. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Antonenkov, V.D.; Hiltunen, J.K. Transfer of metabolites across the peroxisomal membrane. Biochim. Biophys Acta. – Mol. Basis. Dis. 2012. [Google Scholar] [CrossRef]
- Noland, R.C.; Koves, T.R.; Seiler, S.E.; Lum, H.; Lust, R.M.; Ilkayeva, O.; Stevens, R.D.; Hegardt, F.G.; Muoio, D.M. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 2009. [Google Scholar] [CrossRef]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef]
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; De Giorgis, T.; Kiess, W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 2015. [Google Scholar] [CrossRef]
- Yoshino, J.; Baur, J.A.; Imai, S.I. NAD + Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018. [Google Scholar] [CrossRef]
- Bournat, J.C.; Brown, C.W. Mitochondrial Dysfunction in Obesity. Curr. Opin. Endocrino.l Diabetes Obes. 2016. [Google Scholar] [CrossRef]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox. Biol. 2015. [Google Scholar] [CrossRef]
- Loguercio, C.; De Girolamo, V.; De Sio, I.; Tuccillo, C.; Ascione, A.; Baldi, F.; Budillon, G.; Cimino, L.; Di Carlo, A.; Pia Di Marino, M.; et al. Non-alcoholic fatty liver disease in an area of southern italy: Main clinical, histological, and pathophysiological aspects. J. Hepatol. 2001. [Google Scholar] [CrossRef]
- Seki, S.; Kitada, T.; Yamada, T.; Sakaguchi, H.; Nakatani, K.; Wakasa, K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J. Hepatol. 2002. [Google Scholar] [CrossRef]
- Ore, A.; Akinloye, O.A. Oxidative Stress and Antioxidant Biomarkers in Clinical and Experimental Models of Non-Alcoholic Fatty Liver Disease. Medicina 2019, 55, 26. [Google Scholar] [CrossRef]
- Morrison, M.C.; Verschuren, L.; Salic, K.; Verheij, J.; Menke, A.; Wielinga, P.Y.; Iruarrizaga-Lejarreta, M.; Gole, L.; Yu, W.; Turner, S.; et al. Obeticholic Acid Modulates Serum Metabolites and Gene Signatures Characteristic of Human NASH and Attenuates Inflammation and Fibrosis Progression in Ldlr-/-.Leiden Mice. Hepatol. Commun. 2018, 2, 1513–1532. [Google Scholar] [CrossRef]
- Morrison, M.C.; Kleemann, R.; van Koppen, A.; Hanemaaijer, R.; Verschuren, L. Key inflammatory processes in human NASH are reflected in Ldlr-/-.Leiden mice: A translational gene profiling study. Front. Physiol. 2018. [Google Scholar] [CrossRef]
- Van Koppen, A.; Verschuren, L.; van den Hoek, A.M.; Verheij, J.; Morrison, M.C.; Li, K.; Nagabukuro, H.; Costessi, A.; Caspers, M.P.M.; van den Broek, T.J.; et al. Uncovering a Predictive Molecular Signature for the Onset of NASH-Related Fibrosis in a Translational NASH Mouse Model. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 83–98.e10. [Google Scholar] [CrossRef]
- Ishikawa, H.; Takaki, A.; Tsuzaki, R.; Yasunaka, T.; Koike, K.; Shimomura, Y.; Seki, H.; Matsushita, H.; Miyake, Y.; Ikeda, F.; et al. L-carnitine prevents progression of non-alcoholic steatohepatitis in a mouse model with upregulation of mitochondrial pathway. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Melton, S.A.; Keenan, M.J.; Stanciu, C.E.; Hegsted, M.; Zablah-Pimentel, E.M.; O’Neil, C.E.; Gaynor, P.; Schaffhauser, A.; Owen, K.; Prisby, R.D.; et al. L-carnitine supplementation does not promote weight loss in ovariectomized rats despite endurance exercise. Int. J. Vitam. Nutr. Res. 2005. [Google Scholar] [CrossRef]
- Fujisawa, K.; Takami, T.; Matsuzaki, A.; Matsumoto, T.; Yamamoto, N.; Terai, S.; Sakaida, I. Evaluation of the effects of L-carnitine on medaka (Oryzias latipes) fatty liver. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Del Vecchio, F.; Coswig, V.; Galliano, L. Comment on ‘The effect of (L-)carnitine on weight loss in adults: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2016. [Google Scholar] [CrossRef]
- Shi, W.; Hegeman, M.A.; van Dartel, D.A.M.; Tang, J.; Suarez, M.; Swarts, H.; van der Hee, B.; Arola, L.; Keijer, J. Effects of a wide range of dietary nicotinamide riboside (NR) concentrations on metabolic flexibility and white adipose tissue (WAT) of mice fed a mildly obesogenic diet. Mol. Nutr. Food Res. 2017. [Google Scholar] [CrossRef]
- Martens, C.R.; Denman, B.A.; Mazzo, M.R.; Armstrong, M.L.; Reisdorph, N.; McQueen, M.B.; Chonchol, M.; Seals, D.R. Chronic nicotinamide riboside supplementation is well-Tolerated and elevates NAD+ in healthy middle-Aged and older adults. Nat. Commun. 2018. [Google Scholar] [CrossRef]
- Dollerup, O.L.; Christensen, B.; Svart, M.; Schmidt, M.S.; Sulek, K.; Ringgaard, S.; Stødkilde-Jørgensen, H.; Møller, N.; Brenner, C.; Treebak, J.T.; et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: Safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Villani, R.G.; Gannon, J.; Self, M.; Rich, P.A. L-Carnitine Supplementation Combined with Aerobic Training Does Not Promote Weight Loss in Moderately Obese Women. Int. J. Sport. Nutr. Exerc. Metab. 2016. [Google Scholar] [CrossRef]
- Rafraf, M.; Karimi, M.; Jafari, A. Effect of L-carnitine supplementation in comparison with moderate aerobic training on serum inflammatory parameters in healthy obese women. J. Sports Med. Phys. Fitness. 2015, 55, 1363–1370. [Google Scholar]
- Gariani, K.; Menzies, K.J.; Ryu, D.; Wegner, C.J.; Wang, X.; Ropelle, E.R.; Moullan, N.; Zhang, H.; Perino, A.; Lemos, V.; et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology 2016. [Google Scholar] [CrossRef]
- Watanabe, H.; Inaba, Y.; Kimura, K.; Matsumoto, M.; Kaneko, S.; Kasuga, M.; Inoue, H. Sirt2 facilitates hepatic glucose uptake by deacetylating glucokinase regulatory protein. Nat. Commun. 2018. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Gargante, M.P.; Russo, C.; Antic, T.; Vacante, M.; Malaguarnera, M.; Avitabile, T.; Li Volti, G.; Galvano, F. L-carnitine supplementation to diet: A new tool in treatment of nonalcoholic steatohepatitisa randomized and controlled clinical trial. Am. J. Gastroenterol. 2010. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Cattaneo, D.; Loriga, G.; Ledda, F.; Motterlini, N.; Gherardi, G.; Orisio, S.; Remuzzi, G. Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: Effects of acetyl-l-carnitine therapy. Hypertension 2009. [Google Scholar] [CrossRef]
- Cheema, U.B.; Most, E.; Eder, K.; Ringseis, R. Effect of lifelong carnitine supplementation on plasma and tissue carnitine status, hepatic lipid metabolism and stress signalling pathways and skeletal muscle transcriptome in mice at advanced age. Br. J. Nutr. 2019. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Gargante, M.P.; Russo, C.; Antic, T.; Vacante, M.; Malaguarnera, M.; Avitabile, T.; Li Volti, G.; Galvano, F. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 2010. [Google Scholar] [CrossRef]
- Koves, T.R.; Li, P.; An, J.; Akimoto, T.; Slentz, D.; Ilkayeva, O.; Dohm, G.L.; Yan, Z.; Newgard, C.B.; Muoio, D.M. Peroxisome proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J. Biol. Chem. 2005. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008. [Google Scholar] [CrossRef]
- Li, L.O.; Klett, E.L.; Coleman, R.A. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim. Biophys. Acta. – Mol. Cell Biol. Lipids 2010. [Google Scholar] [CrossRef]
- Lehmann, R.; Zhao, X.; Weigert, C.; Simon, P.; Fehrenbach, E.; Fritsche, J.; Machann, J.; Schick, F.; Wang, J.; Hoene, M.; et al. Medium chain acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. PLoS ONE 2010. [Google Scholar] [CrossRef]
- St-Pierre, J.; Lin, J.; Krauss, S.; Tarr, P.T.; Yang, R.; Newgard, C.B.; Spiegelman, B.M. Bioenergetic analysis of peroxisome proliferator-activated receptor γ coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J. Biol. Chem. 2003. [Google Scholar] [CrossRef]
- Cheng, X.; Li, J.; Guo, D. SCAP/SREBPs are Central Players in Lipid Metabolism and Novel Metabolic Targets in Cancer Therapy. Curr. Top Med. Chem. 2018. [Google Scholar] [CrossRef]
- Hu, T.; Foxworthy, P.; Siesky, A.; Ficorilli, J.V.; Gao, H.; Li, S.; Christe, M.; Ryan, T.; Cao, G.; Eacho, P.; et al. Hepatic peroxisomal fatty acid β-oxidation is regulated by liver X receptor α. Endocrinology 2005. [Google Scholar] [CrossRef]
- Choemaker, M.H.; Kleemann, R.; Morrison, M.C.; Verheij, J.; Salic, K.; Van Tol, E.A.F.; Kooistra, T.; Wielinga, P.Y. A casein hydrolysate based formulation attenuates obesity and associated nonalcoholic fatty liver disease and atherosclerosis in LDLr-/-.Leiden mice. PLoS ONE 2017. [Google Scholar] [CrossRef]
- Kühnast, S.; Van Der Tuin, S.J.L.; Van Der Hoorn, J.W.A.; Van Klinken, J.B.; Simic, B.; Pieterman, E.; Havekes, L.M.; Landmesser, U.; Lüscher, T.F.; Van Dijk, K.W.; et al. Anacetrapib reduces progression of atherosclerosis, mainly by reducing non-HDL-cholesterol, improves lesion stability and adds to the beneficial effects of atorvastatin. Eur. Heart J. 2015. [Google Scholar] [CrossRef]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; Van Den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/ electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009. [Google Scholar] [CrossRef]
- Evans, A.; Bridgewater, B.; Liu, Q.; Mitchell, M.; Robinson, R.; Dai, H.; Stewart, S.; DeHaven, C.; Miller, L. High Resolution Mass Spectrometry Improves Data Quantity and Quality as Compared to Unit Mass Resolution Mass Spectrometry in High-Throughput Profiling Metabolomics. J. Postgenomics Drug Biomark Dev. 2015. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010. [Google Scholar] [CrossRef]
- Liang, W.; Tonini, G.; Mulder, P.; Kelder, T.; van Erk, M.; van den Hoek, A.M.; Mariman, R.; Wielinga, P.Y.; Baccini, M.; Kooistra, T.; et al. Coordinated and Interactive Expression of Genes of Lipid Metabolism and Inflammation in Adipose Tissue and Liver during Metabolic Overload. PLoS ONE 2013. [Google Scholar] [CrossRef]




| Chow | HFD | LC | NR | COMBI | |
|---|---|---|---|---|---|
| Cholesterol (mM) | 11.0 ± 3.3 *** | 32.2 ± 14.4 | 28.6 ± 12.5 | 30.3 ± 10.6 | 25.4 ± 10.2 |
| HDL-cholesterol (mM) | 1.2 ± 0.6 *** | 3.2 ± 1.4 | 2.5 ± 1.0 | 2.8 ± 1.3 | 2.5 ± 1.2 |
| Non-HDL cholesterol (mM) | 9.8 ± 3.6 *** | 27.4 ± 12.3 | 26.1 ± 12.3 | 26.1 ± 8.9 | 22.9 ± 9.4 |
| Triglycerides (mM) | 2.6 ± 1.1 ** | 6.1 ± 5.4 | 4.9 ± 2.3 | 5.3 ± 3.9 | 3.7 ± 2.1 |
| Glucose (mM) | 7.6 ± 1.5 | 8.1 ± 2.1 | 7.5 ± 1.1 | 7.9 ± 1.6 | 7.4 ± 1.0 |
| Insulin (ng/mL) | 7.3 ± 7.1 ** | 24.8 ± 21.0 | 21.9 ± 18.7 | 28.0 ± 14.8 | 15.0 ± 7.7 |
| ALT (U/L) | 117 ± 83 *** | 368 ± 249 | 330 ± 132 | 293 ± 127 | 267 ± 142 |
| Acylcarnitines | HFD vs. Chow | LC vs. HFD | NR vs. HFD | COMBI vs. HFD | |
|---|---|---|---|---|---|
| Short-chain | 3-hydroxybutyrylcarnitine (C4-1) | 0.1 | 0.5 | −0.9 | −0.5 |
| 3-hydroxybutyrylcarnitine (C4-2) | −0.6 | 0.6 | −0.3 | 0.3 | |
| valerylcarnitine (C5) | −0.8 | −0.1 | −0.4 | 0.2 | |
| Medium-chain | hexanoylcarnitine (C6) | −0.4 | −0.0 | −0.5 | 0.4 |
| octanoylcarnitine (C8) | −0.2 | 0.3 | −0.5 | 0.4 | |
| decanoylcarnitine (C10) | 0.3 | 0.7 | −0.4 | 0.3 | |
| laurylcarnitine (C12) | 0.2 | 0.3 | −0.5 | 0.3 | |
| cis-4-decenoylcarnitine (C10:1) | 0.2 | 0.4 | −0.5 | 0.4 | |
| 5-dodecenoylcarnitine (C12:1) | 0.3 | 0.3 | −0.6 | 0.1 | |
| Long-chain | myristoylcarnitine (C14) | 0.2 | 0.2 | −0.4 | 0.2 |
| pentadecanoylcarnitine (C15) | 0.8 | 0.2 | −0.4 | 0.1 | |
| palmitoylcarnitine (C16) | 0.8 | 0.1 | −0.3 | −0.1 | |
| margaroylcarnitine (C17) | 1.7 | 0.2 | −0.3 | −0.0 | |
| stearoylcarnitine (C18) | 2.1 | 0.1 | −0.2 | 0.0 | |
| oleoylcarnitine (C18) | 1.5 | 0.0 | −0.4 | −0.3 | |
| arachidoylcarnitine (C20) | 1.8 | 0.4 | −0.1 | 0.2 | |
| myristoleoylcarnitine (C14:1) | 0.5 | 0.3 | −0.5 | 0.2 | |
| palmitoleoylcarnitine (C16:1) | 0.3 | 0.1 | −0.6 | −0.4 | |
| linoleoylcarnitine (C18:2) | 0.8 | 0.2 | −0.4 | −0.1 | |
| linolenoylcarnitine (C18:3) | 0.0 | 0.3 | −0.4 | −0.0 | |
| eicosenoylcarnitine (C20:1) | 2.0 | −0.1 | −0.3 | −0.4 | |
| dihomo-linoleoylcarnitine (C20:2) | 1.4 | 0.1 | −0.3 | −0.1 | |
| arachidonoylcarnitine (C20:4) | 0.6 | 0.4 | −0.4 | 0.1 | |
| dihomo-linolenoylcarnitine (20:3n3 or 6) | 0.9 | 0.2 | −0.3 | 0.0 |
| Upstream Regulator | HFD vs. Chow | LC vs. HFD | NR vs. HFD | COMBI vs. HFD | ||||
|---|---|---|---|---|---|---|---|---|
| Metabolism-related | Activation | p-value | Activation | p-value | Activation | p-value | Activation | p-value |
| z-score | z-score | z-score | z-score | |||||
| ACOX1 | −7.1 | 2.1 × 10−32 | n/a | n/a | n/a | n/a | 2.7 | 7.1 × 10−7 |
| ATP7B | −3.2 | 5.0 × 10−6 | n/a | n/a | n/a | 2.5 × 10−2 | 2.0 | 9.3 × 10−4 |
| INSIG1 | −2.1 | 8.1 × 10−13 | n/a | n/a | n/a | n/a | −1.9 | 2.0 × 10−5 |
| INSR | −2.3 | 6.1 × 10−9 | n/a | n/a | n/a | n/a | 1.7 | 1.4 × 10−5 |
| NR3C1 | −2.1 | 8.9 × 10−18 | n/a | n/a | n/a | n/a | −1.6 | 3.6 × 10−2 |
| PPARGC1B | −3.3 | 3.5 × 10−2 | n/a | n/a | n/a | n/a | 2.2 | 2.4 × 10−4 |
| SCAP | −4.8 | 1.5 × 10−9 | n/a | n/a | n/a | n/a | 3.3 | 1.4 × 10−11 |
| SREBF1 | −1.1 | 2.0 × 10−9 | n/a | n/a | n/a | n/a | 2.7 | 9.7 × 10−10 |
| SREBF2 | −3.0 | 7.2 × 10−9 | n/a | n/a | n/a | n/a | 2.6 | 8.7 × 10−13 |
| SIRT2 | −3.2 | 9.4 × 10−5 | n/a | n/a | n/a | n/a | 2.2 | 1.3 × 10−6 |
| TSC2 | −4.7 | 3.9 × 10−12 | n/a | n/a | n/a | n/a | 2.0 | 8.9 × 10−3 |
| CDKN2A | 0.0 | 2.7 × 10−9 | n/a | n/a | n/a | n/a | −2.4 | 4.0 × 10−2 |
| CNR1 | 2.7 | 7.4 × 10−3 | n/a | n/a | n/a | n/a | n/a | n/a |
| CYP51A1 | 3.0 | 1.8 × 10−5 | n/a | n/a | n/a | n/a | n/a | 3.7 × 10−4 |
| CYP2E1 | 2.1 | 6.2 × 10−6 | n/a | n/a | n/a | n/a | n/a | n/a |
| EP300 | 4.5 | 8.7 × 10−14 | n/a | n/a | n/a | n/a | −2.2 | 2.7 × 10−3 |
| FOXO1 | 4.5 | 4.1 × 10−9 | n/a | n/a | n/a | n/a | −2.1 | 1.5 × 10−2 |
| FOXO3 | 0.6 | 9.2 × 10−7 | n/a | n/a | n/a | n/a | −2.2 | 4.3 × 10−2 |
| MAT1A | 2.4 | 9.0 × 10−2 | n/a | n/a | n/a | n/a | n/a | n/a |
| NR3C2 | 2.1 | 8.9 × 10−7 | n/a | n/a | n/a | n/a | −2.0 | 6.4 × 10−3 |
| TP53 | 2.2 | 1.58 × 10−60 | n/a | n/a | n/a | n/a | −2.0 | 2.0 × 10−3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salic, K.; Gart, E.; Seidel, F.; Verschuren, L.; Caspers, M.; van Duyvenvoorde, W.; Wong, K.E.; Keijer, J.; Bobeldijk-Pastorova, I.; Wielinga, P.Y.; et al. Combined Treatment with L-Carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis. Int. J. Mol. Sci. 2019, 20, 4359. https://doi.org/10.3390/ijms20184359
Salic K, Gart E, Seidel F, Verschuren L, Caspers M, van Duyvenvoorde W, Wong KE, Keijer J, Bobeldijk-Pastorova I, Wielinga PY, et al. Combined Treatment with L-Carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis. International Journal of Molecular Sciences. 2019; 20(18):4359. https://doi.org/10.3390/ijms20184359
Chicago/Turabian StyleSalic, Kanita, Eveline Gart, Florine Seidel, Lars Verschuren, Martien Caspers, Wim van Duyvenvoorde, Kari E. Wong, Jaap Keijer, Ivana Bobeldijk-Pastorova, Peter Y. Wielinga, and et al. 2019. "Combined Treatment with L-Carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis" International Journal of Molecular Sciences 20, no. 18: 4359. https://doi.org/10.3390/ijms20184359
APA StyleSalic, K., Gart, E., Seidel, F., Verschuren, L., Caspers, M., van Duyvenvoorde, W., Wong, K. E., Keijer, J., Bobeldijk-Pastorova, I., Wielinga, P. Y., & Kleemann, R. (2019). Combined Treatment with L-Carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis. International Journal of Molecular Sciences, 20(18), 4359. https://doi.org/10.3390/ijms20184359