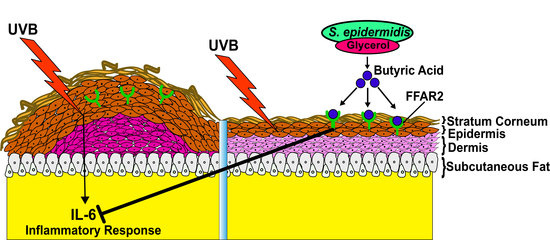
Butyric Acid from Probiotic Staphylococcus epidermidis in the Skin Microbiome Down-Regulates the Ultraviolet-Induced Pro-Inflammatory IL-6 Cytokine via Short-Chain Fatty Acid Receptor
Abstract
:1. Introduction
2. Results
2.1. S. epidermidis Mediates Glycerol Fermentation and Acetolactate Synthase (ALS) Activity
2.2. Butyric Acid, a Product in Fermented Media upon Glycerol Fermentation by S. epidermidis
2.3. Mixture of S. epidermidis and Glycerol or Butyric Acid alone Inhibited UVB Induced Skin Inflammation
2.4. Knocking Down Free Fatty Acid Receptor 2 (FFAR2) Inhibited Butyric Acid Mediated Attenuation of Inflammation in Chronic UVB
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Chemicals
4.3. Bacterial Culture
4.4. Fermentation of Bacteria
4.5. Inhibition of ALS Activity by Furfural
4.6. Ultraviolet Light Exposure
4.7. Wound Measurement
4.8. Histological Analysis
4.9. siRNA-Mediated Gene Silencing of GPR43/FFAR2
4.10. Drug Treatment
4.11. ELISA
4.12. HPLC
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lopez-Camarillo, C.; Ocampo, E.A.; Casamichana, M.L.; Perez-Plasencia, C.; Alvarez-Sanchez, E.; Marchat, L.A. Protein kinases and transcription factors activation in response to UV-radiation of skin: Implications for carcinogenesis. Int. J. Mol. Sci. 2012, 13, 142–172. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B 2001, 63, 8–18. [Google Scholar] [CrossRef]
- De Fabo, E.C.; Noonan, F.P. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J. Exp. Med. 1983, 158, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Sakaguchi, I. Protection of human keratinocytes from UVB-induced inflammation using root extract of Lithospermum erythrorhizon. Biol. Pharm. Bull. 2007, 30, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Ansel, J.C.; Luger, T.A.; Green, I. The effect of in vitro and in vivo UV irradiation on the production of ETAF activity by human and murine keratinocytes. J. Investig. Dermatol. 1983, 81, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Kirnbauer, R.; Kock, A.; Neuner, P.; Forster, E.; Krutmann, J.; Urbanski, A.; Schauer, E.; Ansel, J.C.; Schwarz, T.; Luger, T.A. Regulation of epidermal cell interleukin-6 production by UV light and corticosteroids. J. Investig. Dermatol. 1991, 96, 484–489. [Google Scholar] [CrossRef]
- Acker, T.; Fandrey, J.; Acker, H. The good, the bad and the ugly in oxygen-sensing: ROS, cytochromes and prolyl-hydroxylases. Cardiovasc. Res. 2006, 71, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloos, W.E.; Musselwhite, M.S. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl. Microbiol. 1975, 30, 381–385. [Google Scholar] [PubMed]
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.; Di Nardo, A.; Nakatsuji, T.; Leichtle, A.; Yang, Y.; Cogen, A.L.; Wu, Z.R.; Hooper, L.V.; Schmidt, R.R.; von Aulock, S.; et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 2009, 15, 1377–1382. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.-J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Cogen, A.L.; Yamasaki, K.; Sanchez, K.M.; Dorschner, R.A.; Lai, Y.; MacLeod, D.T.; Torpey, J.W.; Otto, M.; Nizet, V.; Kim, J.E.; et al. Selective Antimicrobial Action Is Provided by Phenol-Soluble Modulins Derived from Staphylococcus epidermidis, a Normal Resident of the Skin. J. Investig. Dermatol. 2010, 130, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; Two, A.; Gallo, R.L.; Huang, C.M. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: Implications of probiotics in acne vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Myagmardoloonjin, B.; Keshari, S.; Negari, I.P.; Huang, C.M. 5-Methyl Furfural Reduces the Production of Malodors by Inhibiting Sodium l-Lactate Fermentation of Staphylococcus epidermidis: Implication for Deodorants Targeting the Fermenting Skin Microbiome. Microorganisms 2019, 7, 239. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.J.; Marito, S.; Yang, J.J.; Keshari, S.; Chew, C.H.; Chen, C.C.; Huang, C.M. A Microtube Array Membrane (MTAM) Encapsulated Live Fermenting Staphylococcus epidermidis as a Skin Probiotic Patch against Cutibacterium acnes. Int. J. Mol. Sci. 2018, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Malav, M.K.; Prasad, S.; Kharia, S.K.; Kumar, S.; Sheetal, K.; Kannojiya, S. Furfural and 5-HMF: Potent fermentation inhibitors and their removal techniques. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2060–2066. [Google Scholar] [CrossRef]
- Carvalho, S.M.; de Jong, A.; Kloosterman, T.G.; Kuipers, O.P.; Saraiva, L.M. The Staphylococcus aureus α-Acetolactate Synthase ALS Confers Resistance to Nitrosative Stress. Front. Microbiol. 2017, 8, 1273. [Google Scholar] [CrossRef] [PubMed]
- Härtig, E.; Jahn, D. Regulation of the Anaerobic Metabolism in Bacillus subtilis. Adv. Microb. Physiol. 2012, 61, 195–216. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 33, 25–33. [Google Scholar] [CrossRef]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Kao, M.S.; Huang, S.; Chang, W.L.; Hsieh, M.F.; Huang, C.J.; Gallo, R.L.; Huang, C.M. Microbiome precision editing: Using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Song, X.; Bi, Z.; Chu, W.; Wan, Y. UV-induced NF-kappaB activation and expression of IL-6 is attenuated by (-)-epigallocatechin-3-gallate in cultured human keratinocytes in vitro. Int. J. Mol. Med. 2005, 16, 943–950. [Google Scholar] [PubMed]
- Smerdon, M.J.; Lan, S.Y.; Calza, R.E.; Reeves, R. Sodium butyrate stimulates DNA repair in UV-irradiated normal and xeroderma pigmentosum human fibroblasts. J. Biol. Chem. 1982, 257, 13441–13447. [Google Scholar] [PubMed]
- Xiong, F.; Mou, Y.Z.; Xiang, X.Y. Inhibition of mouse B16 melanoma by sodium butyrate correlated to tumor associated macrophages differentiation suppression. Int. J. Clin. Exp. Med. 2015, 8, 4170–4174. [Google Scholar] [PubMed]
- Yoshizumi, M.; Nakamura, T.; Kato, M.; Ishioka, T.; Kozawa, K.; Wakamatsu, K.; Kimura, H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol. Int. 2008, 32, 1405–1411. [Google Scholar] [CrossRef]
- Pirozzi, C.; Francisco, V.; Guida, F.D.; Gomez, R.; Lago, F.; Pino, J.; Meli, R.; Gualillo, O. Butyrate Modulates Inflammation in Chondrocytes via GPR43 Receptor. Cell Physiol. Biochem. 2018, 51, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Masui, R.; Sasaki, M.; Funaki, Y.; Ogasawara, N.; Mizuno, M.; Iida, A.; Izawa, S.; Kondo, Y.; Ito, Y.; Tamura, Y.; et al. G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm. Bowel Dis. 2013, 19, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE 2003, 2003. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-S.; Kundu, J.K.; Chun, K.-S.; Na, H.-K.; Surh, Y.-J. Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential targets. Arch. Biochem. Biophys. 2014, 559, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.H.; Thygesen, A.; Thomsen, A.B. Identification and characterization of fermentation inhibitors formed during hydrothermal treatment and following SSF of wheat straw. Appl. Microbiol. Biotechnol. 2009, 83, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Perrine, S.P.; Dover, G.H.; Daftari, P.; Walsh, C.T.; Jin, Y.; Mays, A.; Faller, D.V. Isobutyramide, an orally bioavailable butyrate analogue, stimulates fetal globin gene expression in vitro and in vivo. Br. J. Haematol. 1994, 88, 555–561. [Google Scholar] [CrossRef]
- Vassilev, I.; Hernandez, P.A.; Batlle-Vilanova, P.; Freguia, S.; Krömer, J.O.; Keller, J.; Ledezma, P.; Virdis, B. Microbial Electrosynthesis of Isobutyric, Butyric, Caproic Acids, and Corresponding Alcohols from Carbon Dioxide. ACS Sustain. Chem. Eng. 2018, 6, 8485–8493. [Google Scholar] [CrossRef]
- Kober, M.-M.; Bowe, W.P. The effect of probiotics on immune regulation, acne, and photoaging. Int. J. Women’s Dermatol. 2015, 1, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Guéniche, A.; Benyacoub, J.; Buetler, T.M.; Smola, H.; Blum, S. Supplementation with oral probiotic bacteria maintains cutaneous immune homeostasis after UV exposure. Eur. J. Dermatol. 2006, 16, 511–517. [Google Scholar]
- Cope, E.K.; Lynch, S.V. Novel microbiome-based therapeutics for chronic rhinosinusitis. Curr. Allergy Asthma Rep. 2015, 15, 9. [Google Scholar] [CrossRef]
- Agarwal, R.; Katiyar, S.K.; Khan, S.G.; Mukhtar, H. Protection against ultraviolet B radiation-induced effects in the skin of SKH-1 hairless mice by a polyphenolic fraction isolated from green tea. Photochem. Photobiol. 1993, 58, 695–700. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Ross, M.S.; Parrett, M.L.; Oberyszyn, T.M. Topical application of a selective cyclooxygenase inhibitor suppresses UVB mediated cutaneous inflammation. Prostaglandins Other Lipid Mediat. 2000, 62, 367–384. [Google Scholar] [CrossRef]
- Borycka-Kiciak, K.; Banasiewicz, T.; Rydzewska, G. Butyric acid—A well-known molecule revisited. Prz. Gastroenterol. 2017, 12, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The Short-Chain Fatty Acid Sodium Butyrate Functions as a Regulator of the Skin Immune System. J. Investig. Dermatol. 2017, 137, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.P.; Mehrotra, N.K. Differential effects of butyric acid on mouse skin tumorigenesis. Biomed. Env. Sci. 1997, 10, 436–441. [Google Scholar]
- Bruhs, A.; Schwarz, T.; Schwarz, A. 325 The short chain fatty acid sodium butyrate attenuates imiquimod-induced psoriasis-like skin inflammation. J. Investig. Dermatol. 2017, 137, S248. [Google Scholar] [CrossRef]
- Karaki, S.; Mitsui, R.; Hayashi, H.; Kato, I.; Sugiya, H.; Iwanaga, T.; Furness, J.B.; Kuwahara, A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006, 324, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Krejner, A.; Bruhs, A.; Mrowietz, U.; Wehkamp, U.; Schwarz, T.; Schwarz, A. Decreased expression of G-protein-coupled receptors GPR43 and GPR109a in psoriatic skin can be restored by topical application of sodium butyrate. Arch. Dermatol. Res. 2018, 310, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz, B.A. The Role of Free Radicals, Iron, and Antioxidants in Ultraviolet Radiation-Induced Skin Damage. Available online: https://www.healthcare.uiowa.edu/CoreFacilities/esr/education/theses/pdf/Jurkiewicz-PhD-1995.pdf (accessed on 11 August 2019).
- Cogan, T.M.; Fitzgerald, R.J.; Doonan, S. Acetolactate synthase of Leuconostoc lactis and its regulation of acetoin production. J. Dairy Res. 2009, 51, 597–604. [Google Scholar] [CrossRef]
- Pan, J.; Ruan, W.; Qin, M.; Long, Y.; Wan, T.; Yu, K.; Zhai, Y.; Wu, C.; Xu, Y. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci. Rep. 2018, 8, 1117. [Google Scholar] [CrossRef]
- Dou, S.; Yao, Y.-D.; Yang, X.-Z.; Sun, T.-M.; Mao, C.-Q.; Song, E.-W.; Wang, J. Anti-Her2 single-chain antibody mediated DNMTs-siRNA delivery for targeted breast cancer therapy. J. Control. Release 2012, 161, 875–883. [Google Scholar] [CrossRef]
- Pizzonero, M.; Dupont, S.; Babel, M.; Beaumont, S.; Bienvenu, N.; Blanque, R.; Cherel, L.; Christophe, T.; Crescenzi, B.; De Lemos, E.; et al. Discovery and optimization of an azetidine chemical series as a free fatty acid receptor 2 (FFA2) antagonist: From hit to clinic. J. Med. Chem. 2014, 57, 10044–10057. [Google Scholar] [CrossRef]
- Keshari, S.; Sipayung, A.D.; Hsieh, C.C.; Su, L.J.; Chiang, Y.R.; Chang, H.C.; Yang, W.C.; Chuang, T.H.; Chen, C.L.; Huang, C.M. The IL-6/p-BTK/p-ERK signaling mediates the calcium phosphate-induced pruritus. FASEB J. 2019. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keshari, S.; Balasubramaniam, A.; Myagmardoloonjin, B.; Herr, D.R.; Negari, I.P.; Huang, C.-M. Butyric Acid from Probiotic Staphylococcus epidermidis in the Skin Microbiome Down-Regulates the Ultraviolet-Induced Pro-Inflammatory IL-6 Cytokine via Short-Chain Fatty Acid Receptor. Int. J. Mol. Sci. 2019, 20, 4477. https://doi.org/10.3390/ijms20184477
Keshari S, Balasubramaniam A, Myagmardoloonjin B, Herr DR, Negari IP, Huang C-M. Butyric Acid from Probiotic Staphylococcus epidermidis in the Skin Microbiome Down-Regulates the Ultraviolet-Induced Pro-Inflammatory IL-6 Cytokine via Short-Chain Fatty Acid Receptor. International Journal of Molecular Sciences. 2019; 20(18):4477. https://doi.org/10.3390/ijms20184477
Chicago/Turabian StyleKeshari, Sunita, Arun Balasubramaniam, Binderiya Myagmardoloonjin, Deron Raymond Herr, Indira Putri Negari, and Chun-Ming Huang. 2019. "Butyric Acid from Probiotic Staphylococcus epidermidis in the Skin Microbiome Down-Regulates the Ultraviolet-Induced Pro-Inflammatory IL-6 Cytokine via Short-Chain Fatty Acid Receptor" International Journal of Molecular Sciences 20, no. 18: 4477. https://doi.org/10.3390/ijms20184477
APA StyleKeshari, S., Balasubramaniam, A., Myagmardoloonjin, B., Herr, D. R., Negari, I. P., & Huang, C. -M. (2019). Butyric Acid from Probiotic Staphylococcus epidermidis in the Skin Microbiome Down-Regulates the Ultraviolet-Induced Pro-Inflammatory IL-6 Cytokine via Short-Chain Fatty Acid Receptor. International Journal of Molecular Sciences, 20(18), 4477. https://doi.org/10.3390/ijms20184477