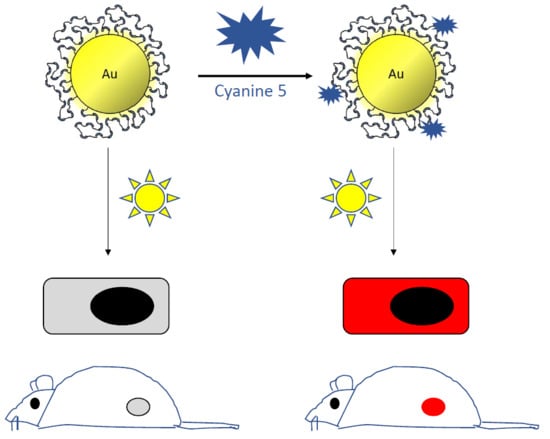
Fluorescent Radiosensitizing Gold Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Fluorescent Gold Nanoparticles
2.2. Internalization of the Au@DTDTPA-Cy5 Nanoparticles Monitored by Fluorescence Imaging
2.3. In Vivo Fluorescence Imaging
3. Materials and Methods
3.1. Au@DTDTPA Synthesis
3.2. Functionalization of Au@DTDTPA by NIR Organic Dye Cyanine-5-Amine (Cy5-NH2)
3.3. Transmission Electron Microscopy
3.4. Measurements of ζ-Potential
3.5. Fluorescence Spectrometry
3.6. UV-Visible Spectrophotometry
3.7. Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES)
3.8. Centrifugation
3.9. Cell Culture
3.10. High-Angle Annular Dark-Field Scanning Transmission Electron Microscopy (HAADF-STEM)
3.11. Confocal Microscopy
3.12. Animal Models
3.13. In Vivo and Ex Vivo Fluorescence Imaging
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Au@DTDTPA | gold nanoparticles coated by linear chelator |
| Au@DTDTPA-Cy5 | gold nanoparticles coated by linear chelator and functionalized with cyanine 5 |
| DTPA | diethylenetriaminepentaacetic acid (linear chelator) |
| DTDTPA | dithiolated derivative of diethylenetriaminepentaacetic acid |
| Au@TADOTAGA | gold nanoparticles coated by macrocyclic chelator |
| DOTA | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DOTAGA | 1,4,7,10-tetraazacyclododecan-1-glutaric acid-4,7,10-triacetic acid |
| TADOTAGA | DOTAGA functionalized by thioctic acid |
| Cy5-NH2 | aminated derivative of cyanine 5 |
| Z | atomic number |
| vs. | versus |
| MRI | magnetic resonance imaging |
| SPECT | single-photon emission computed-tomography |
| MRT | microbeam radiation therapy |
| pi | post-injection |
| EDC | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide |
| NHS | N-hydroxysuccinimide |
| MWCO | molecular weight cut-off |
| TEM | transmission electron microscopy |
| UV | ultraviolet |
| ICP-OES | inductively coupled plasma-optical emission spectrometry |
References
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Smilowitz, H.M.; O’Connor, M.J.; Dilmanian, F.A.; Slatkin, D.N. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedecine 2013, 8, 1601–1609. [Google Scholar] [CrossRef] [Green Version]
- Schuemann, J.; Berbeco, R.; Chithrani, D.B.; Cho, S.H.; Kumar, R.; McMahon, S.J.; Sridhar, S.; Krishnan, S. Roadmap to clinical use of gold nanoparticles for radiation sensitization. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 189–205. [Google Scholar] [CrossRef]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nano 2016, 7, 8. [Google Scholar] [CrossRef]
- Rosa, S.; Connolly, C.; Schettino, G.; Butterworth, K.T.; Prise, K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nano 2017, 8, 2. [Google Scholar] [CrossRef]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2018, 63, 02TR01. [Google Scholar] [CrossRef]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Usami, N.; Porcel, E.; Lacombe, S.; Le Sech, C. Enhancement of radiation effect by heavy elements. Mutat. Res. 2010, 704, 123–131. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for radiation therapy enhancement: The key parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Li, F.; Jin, X.; Li, J.; Chen, W.; Li, Q. Metal-based nanoenhancers for future radiotherapy: Radiosensitizing and synergistic effects on tumor cells. Theranostics 2018, 8, 1824–1849. [Google Scholar] [CrossRef]
- Scheinberg, D.A.; Villa, C.H.; Escorcia, F.E.; McDevitt, M.R. Conscripts of the infinite armada: Systemic cancer therapy using nanomaterials. Nat. Rev. Clin. Oncol. 2010, 7, 266–276. [Google Scholar] [CrossRef]
- Min, Y.; Caster, J.M.; Eblan, M.J.; Wang, A.Z. Clinical translation of nanomedicine. Chem. Rev. 2015, 115, 11147–11190. [Google Scholar] [CrossRef]
- Kunjachan, S.; Ehling, J.; Storm, G.; Kiessling, F.; Lammers, T. Noninvasive imaging of nanomedicines and nanotheranostics: Principles, progress, and prospects. Chem. Rev. 2015, 115, 10907–10937. [Google Scholar] [CrossRef]
- Dou, Y.; Guo, Y.; Li, X.; Li, X.; Wang, S.; Wang, L.; Lv, G.; Zhang, X.; Wang, H.; Gong, X.; et al. Size-tuning ionization to optimize gold nanoparticles for simultaneous enhanced CT imaging and radiotherapy. ACS Nano 2016, 10, 2536–2548. [Google Scholar] [CrossRef]
- Porcel, E.; Liehn, S.; Remita, H.; Usami, N.; Kobayashi, K.; Furusawa, Y.; Le Sech, C.; Lacombe, S. Platinum nanoparticles: A promising material for future cancer therapy? Nanotechnology 2010, 21, 085103. [Google Scholar] [CrossRef]
- Le Duc, G.; Miladi, I.; Alric, C.; Mowat, P.; Bräuer-Krisch, E.; Bouchet, A.; Khalil, E.; Billotey, C.; Janier, M.; Lux, F.; et al. Toward an image-guided microbeam radiation therapy using gadolinium-based nanoparticles. ACS Nano 2011, 5, 9566–9574. [Google Scholar] [CrossRef]
- Dufort, S.; Le Duc, G.; Salomé, M.; Bentivegna, V.; Sancey, L.; Bräuer-Krisch, E.; Requardt, H.; Lux, F.; Coll, J.-L.; Perriat, P.; et al. The high radiosensitizing efficiency of a trace of gadolinium-based nanoparticles in tumors. Sci. Rep. 2016, 6, 29678. [Google Scholar] [CrossRef]
- Lux, F.; Mignot, A.; Mowat, P.; Louis, C.; Dufort, S.; Bernhard, C.; Denat, F.; Boschetti, F.; Brunet, C.; Antoine, R.; et al. Ultrasmall rigid particles as multimodal probes for medical applications. Angew. Chem. Int. Ed. 2011, 50, 12299–12303. [Google Scholar] [CrossRef]
- Alqathanni, M.; Blencowe, A.; Geso, M.; Ibbott, G. Quantitative 3D Determination of Radiosensitization by Bismuth-Based Nanoparticles. J. Biomed. Nanotechnol. 2016, 12, 464–471. [Google Scholar] [CrossRef]
- Detappe, A.; Thomas, E.; Tibbitt, M.W.; Kunjachan, S.; Zavidij, O.; Parnandi, N.; Reznichenko, E.; Lux, F.; Tillement, O.; Berbeco, R. Ultrasmall silica-based bismuth gadolinium nanoparticles for dual magnetic resonance–computed tomography image guided radiation therapy. Nano Lett. 2017, 17, 1733–1740. [Google Scholar] [CrossRef]
- Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef]
- Debouttière, P.-J.; Roux, S.; Vocanson, F.; Billotey, C.; Beuf, O.; Favre-Réguillon, A.; Lin, Y.; Pellet-Rostaing, S.; Lamartine, R.; Perriat, P.; et al. Design of gold nanoparticles for magnetic resonance imaging. Adv. Funct. Mater. 2006, 16, 2330–2339. [Google Scholar] [CrossRef]
- Alric, C.; Taleb, J.; Le Duc, G.; Mandon, C.; Billotey, C.; Le Meur-Herland, A.; Brochard, T.; Vocanson, F.; Janier, M.; Perriat, P.; et al. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J. Am. Chem. Soc. 2008, 130, 5908–5915. [Google Scholar] [CrossRef]
- Arifin, D.R.; Long, C.M.; Gilad, A.A.; Alric, C.; Roux, S.; Tillement, O.; Link, T.W.; Arepally, A.; Bulte, J.W.M. Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology 2011, 260, 790–798. [Google Scholar] [CrossRef]
- Alric, C.; Miladi, I.; Kryza, D.; Taleb, J.; Lux, F.; Bazzi, R.; Billotey, C.; Janier, M.; Perriat, P.; Roux, S.; et al. The biodistribution of gold nanoparticles designed for renal clearance. Nanoscale 2013, 5, 5930–5939. [Google Scholar] [CrossRef]
- Miladi, I.; Alric, C.; Dufort, S.; Mowat, P.; Dutour, A.; Mandon, C.; Laurent, G.; Bräuer-Krisch, E.; Herath, N.; Coll, J.-L.; et al. The in vivo radiosensitizing effect of gold nanoparticles based MRI contrast agents. Small 2014, 10, 1116–1124. [Google Scholar] [CrossRef]
- Laurent, G.; Bernhard, C.; Dufort, S.; Jiménez Sánchez, G.; Bazzi, R.; Boschetti, F.; Moreau, M.; Vu, T.H.; Collin, B.; Oudot, A.; et al. Minor changes in the macrocyclic ligands but major consequences on the efficiency of gold nanoparticles designed for radiosensitization. Nanoscale 2016, 8, 12054–12065. [Google Scholar] [CrossRef]
- Butterworth, K.T.; Nicol, J.R.; Ghita, M.; Rosa, S.; Chaudhary, P.; McGarry, C.K.; McCarthy, H.O.; Jiménez Sánchez, G.; Bazzi, R.; Roux, S.; et al. Preclinical evaluation of gold-DTDTPA nanoparticles as theranostic agents in prostate cancer radiotherapy. Nanomedicine 2016, 11, 2035–2047. [Google Scholar] [CrossRef] [Green Version]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Miyawaki, A. Visualization of the spatial and temporal dynamics of intracellular signaling. Dev. Cell 2003, 4, 295–305. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Chen, S.; Kimura, K. Synthesis and characterization of carboxylate-modified gold nanoparticle powders dispersible in water. Langmuir 1999, 15, 1075–1082. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R. On the Ligand’s Role in the Fluorescence of Gold Nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef]
- Duff, D.G.; Baiker, A.; Edwards, P.P. A new hydrosol of gold clusters. J. Chem. Soc. Chem. Commun. 1993, 96–98. [Google Scholar] [CrossRef]
- Faure, A.-C.; Dufort, S.; Josserand, V.; Perriat, P.; Coll, J.-L.; Roux, S.; Tillement, O. Control of the in vivo Biodistribution of Hybrid Nanoparticles with Different Poly(ethylene glycol) Coatings. Small 2009, 5, 2565–2575. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef]
- Brust, M.; Fink, J.; Bethell, D.; Schiffrin, D.J.; Kiely, C. Synthesis and reactions of functionalised gold nanoparticles. J. Chem. Soc. Chem. Commun. 1995, 1655–1656. [Google Scholar] [CrossRef]













© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez Sánchez, G.; Maury, P.; Stefancikova, L.; Campion, O.; Laurent, G.; Chateau, A.; Bouraleh Hoch, F.; Boschetti, F.; Denat, F.; Pinel, S.; et al. Fluorescent Radiosensitizing Gold Nanoparticles. Int. J. Mol. Sci. 2019, 20, 4618. https://doi.org/10.3390/ijms20184618
Jiménez Sánchez G, Maury P, Stefancikova L, Campion O, Laurent G, Chateau A, Bouraleh Hoch F, Boschetti F, Denat F, Pinel S, et al. Fluorescent Radiosensitizing Gold Nanoparticles. International Journal of Molecular Sciences. 2019; 20(18):4618. https://doi.org/10.3390/ijms20184618
Chicago/Turabian StyleJiménez Sánchez, Gloria, Pauline Maury, Lenka Stefancikova, Océane Campion, Gautier Laurent, Alicia Chateau, Farhan Bouraleh Hoch, Frédéric Boschetti, Franck Denat, Sophie Pinel, and et al. 2019. "Fluorescent Radiosensitizing Gold Nanoparticles" International Journal of Molecular Sciences 20, no. 18: 4618. https://doi.org/10.3390/ijms20184618
APA StyleJiménez Sánchez, G., Maury, P., Stefancikova, L., Campion, O., Laurent, G., Chateau, A., Bouraleh Hoch, F., Boschetti, F., Denat, F., Pinel, S., Devy, J., Porcel, E., Lacombe, S., Bazzi, R., & Roux, S. (2019). Fluorescent Radiosensitizing Gold Nanoparticles. International Journal of Molecular Sciences, 20(18), 4618. https://doi.org/10.3390/ijms20184618