Effect of Polymer Phase Transition Behavior on Temperature-Responsive Polymer-Modified Liposomes for siRNA Transfection
Abstract
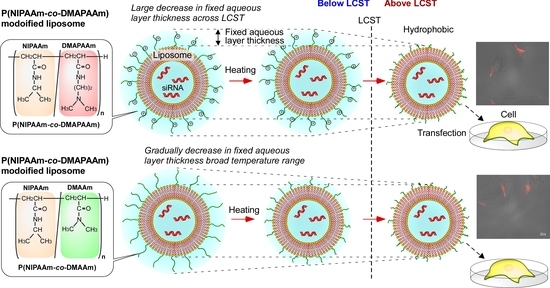
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Prepared Polymers
2.2. Characterization of the Prepared Liposomes
2.3. Cellular Uptake of Liposomes
2.4. Gene Silencing Activity of the Prepared Liposomes
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Thermoresponsive Copolymers
3.3. Characterization of Synthesized Polymers
3.4. Conjugation of Thermoresponsive Polymer to Lipid
3.5. Preparation of Thermoresponsive Polymer-Modified Liposomes
3.6. Liposome Characterization
3.7. Cell Culture
3.8. Gene Silencing of Luciferase Using siRNA Loaded Liposomes
3.9. Suppression of VEGF Using siRNA Loaded Liposomes
3.10. Determination of Cellular Uptake by Fluorescence Microscopy
3.11. Cell Viability Assessment
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIBN | 2,2-Azobisisobutyronitrile |
| BTB | Bromothymol blue |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DMAAm | N,N-Dimethylacrylamide |
| DMAPAAm | N,N-Dimethylaminopropyl acrylamide |
| DMEM | Dulbecco’s modified eagle medium |
| DMF | N,N-dimethylformamide |
| DOTAP | 1,2-Dioleoyl-3-trimethylammonium propane |
| DOPE | L-α-phosphatidyl ethanolamine, dioleoyl |
| SPE | L-α-distearoyl-phosphatidylethanolamine |
| FALT | Fixed aqueous layer thickness |
| GPC | Gel permeation chromatography |
| LCST | Lower critical solution temperature |
| MEM | Minimum essential media |
| NEAA | Non-Essential Amino Acid Solution |
| NIPAAm | N-isopropylacrylamide |
| NMR | Nuclear magnetic resonance |
| PEG | Polyethylene glycol |
| siRNA | Small interfering RNA |
| VEGF | Vascular endothelial growth factor |
References
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Tuschl, T.; Zamore, P.D.; Lehmann, R.; Bartel, D.P.; Sharp, P.A. Targeted mRNA degradation by double-stranded RNA in vitro. Genes Dev. 1999, 13, 3191–3197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamore, P.D.; Tuschl, T.; Sharp, P.A.; Bartel, D.P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000, 101, 25–33. [Google Scholar] [CrossRef]
- Reynolds, A.; Leake, D.; Boese, Q.; Scaringe, S.; Marshall, W.S.; Khvorova, A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004, 22, 326–330. [Google Scholar] [CrossRef]
- Amoozgar, Z.; Yeo, Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 219–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vllasaliu, D.; Fowler, R.; Stolnik, S. PEGylated nanomedicines: Recent progress and remaining concerns. Expert Opin. Drug Deliv. 2014, 11, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhou, Z.; Tang, H.; Song, S.; Peng, J.; Xu, Y. Impact of PEGylation on biodistribution and tumor accumulation of Lipid-Mu peptide-DNA. J. Liposome Res. 2013, 23, 1–10. [Google Scholar] [CrossRef]
- Kono, K.; Nakai, R.; Morimoto, K.; Takagishi, T. Temperature-dependent interaction of thermo-sensitive polymer-modified liposomes with CV1 cells. FEBS Lett. 1999, 456, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Kono, K.; Ozawa, T.; Yoshida, T.; Ozaki, F.; Ishizaka, Y.; Maruyama, K.; Kojima, C.; Harada, A.; Aoshima, S. Highly temperature-sensitive liposomes based on a thermosensitive block copolymer for tumor-specific chemotherapy. Biomaterials 2010, 31, 7096–7105. [Google Scholar] [CrossRef]
- Wang, J.; Ayano, E.; Maitani, Y.; Kanazawa, H. Tunable Surface Properties of Temperature-Responsive Polymer-Modified Liposomes Induce Faster Cellular Uptake. ACS Omega 2017, 2, 316–325. [Google Scholar] [CrossRef]
- Wang, J.; Ayano, E.; Maitani, Y.; Kanazawa, H. Enhanced cellular uptake and gene silencing activity of siRNA using temperature-responsive polymer-modified liposome. Int. J. Pharm. 2017, 523, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Cammas, S.; Suzuki, K.; Sone, C.; Sakurai, Y.; Kataoka, K.; Okano, T. Thermo-responsive polymer nanoparticles with a core-shell micelle structure as site-specific drug carriers. J. Control. Release 1997, 48, 157–164. [Google Scholar] [CrossRef]
- Akimoto, J.; Nakayama, M.; Okano, T. Temperature-responsive polymeric micelles for optimizing drug targeting to solid tumors. J. Control. Release 2014, 193, 2–8. [Google Scholar] [CrossRef]
- Nakayama, M.; Akimoto, J.; Okano, T. Polymeric micelles with stimuli-triggering systems for advanced cancer drug targeting. J. Drug Target. 2014, 22, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, H.; Yamamoto, K.; Matsushima, Y.; Takai, N.; Kikuchi, A.; Sakurai, Y.; Okano, T. Temperature-Responsive Chromatography Using Poly(N-isopropylacrylamide)-Modified Silica. Anal. Chem. 1996, 68, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Okano, T. Temperature-responsive, polymer-modified surfaces for green chromatography. Macromol. Symp. 2004, 207, 217–228. [Google Scholar] [CrossRef]
- Nagase, K.; Kobayashi, J.; Okano, T. Temperature-responsive intelligent interfaces for biomolecular separation and cell sheet engineering. J. R. Soc. Interface 2009, 6 (Suppl. 3), S293–S309. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, H.; Okano, T. Temperature-responsive chromatography for the separation of biomolecules. J. Chromatogr. A 2011, 1218, 8738–8747. [Google Scholar] [CrossRef]
- Nagase, K.; Okano, T. Thermoresponsive-polymer-based materials for temperature-modulated bioanalysis and bioseparations. J. Mater. Chem. B 2016, 4, 6381–6397. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Maeda, M. Temperature-Responsive Formation of Colloidal Nanoparticles from Poly(N-isopropylacrylamide) Grafted with Single-Stranded DNA. Langmuir 2003, 20, 313–319. [Google Scholar] [CrossRef]
- Lai, J.J.; Hoffman, J.M.; Ebara, M.; Hoffman, A.S.; Estournès, C.; Wattiaux, A.; Stayton, P.S. Dual Magnetic-/Temperature-Responsive Nanoparticles for Microfluidic Separations and Assays. Langmuir 2007, 23, 7385–7391. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M.; Ohshima, M.; Hiruta, Y.; Nishimura, T.; Nagase, K.; Kanazawa, H. LAT1-Targeting Thermoresponsive Fluorescent Polymer Probes for Cancer Cell Imaging. Int. J. Mol. Sci. 2018, 19, 1646. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol. Chem. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin Poly(N-isopropylacrylamide) Grafted Layer on Polystyrene Surfaces for Cell Adhesion/Detachment Control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakayama, M.; Yamato, M.; Okano, T. Controlled Chain Length and Graft Density of Thermoresponsive Polymer Brushes for Optimizing Cell Sheet Harvest. Biomacromolecules 2010, 11, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Watanabe, M.; Kikuchi, A.; Yamato, M.; Okano, T. Thermo-Responsive Polymer Brushes as Intelligent Biointerfaces: Preparation via ATRP and Characterization. Macromol. Biosci. 2011, 11, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef]
- Nagase, K.; Okano, T.; Kanazawa, H. Poly(N-isopropylacrylamide) based thermoresponsive polymer brushes for bioseparation, cellular tissue fabrication, and nano actuators. Nano-Struct. Nano-Objects 2018, 16, 9–23. [Google Scholar] [CrossRef]
- Akimoto, A.; Niitsu, E.; Nagase, K.; Okano, T.; Kanazawa, H.; Yoshida, R. Mesenchylmal Stem Cell Culture on Poly(N-isopropylacrylamide) Hydrogel with Repeated Thermo-Stimulation. Int. J. Mol. Sci. 2018, 19, 1253. [Google Scholar] [CrossRef]
- Kobayashi, J.; Arisaka, Y.; Yui, N.; Akiyama, Y.; Yamato, M.; Okano, T. Effect of Temperature Changes on Serum Protein Adsorption on Thermoresponsive Cell-Culture Surfaces Monitored by A Quartz Crystal Microbalance with Dissipation. Int. J. Mol. Sci. 2018, 19, 1516. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-isopropylacrylamide). J. Macromol. Sci. A 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Stayton, P.S. Conjugates of stimuli-responsive polymers and proteins. Prog. Polym. Sci. 2007, 32, 922–932. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Takei, Y.G.; Aoki, T.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Temperature-responsive bioconjugates. 2. Molecular design for temperature-modulated bioseparations. Bioconj. Chem. 1993, 4, 341–346. [Google Scholar] [CrossRef]
- Okano, T.; Bae, Y.H.; Jacobs, H.; Kim, S.W. Thermally on-off switching polymers for drug permeation and release. J. Control. Release 1990, 11, 255–265. [Google Scholar] [CrossRef]
- Akimoto, J.; Nakayama, M.; Sakai, K.; Okano, T. Molecular design of outermost surface functionalized thermoresponsive polymeric micelles with biodegradable cores. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7127–7137. [Google Scholar] [CrossRef]
- Akimoto, J.; Nakayama, M.; Sakai, K.; Okano, T. Temperature-Induced Intracellular Uptake of Thermoresponsive Polymeric Micelles. Biomacromolecules 2009, 10, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, J.; Nakayama, M.; Sakai, K.; Okano, T. Thermally Controlled Intracellular Uptake System of Polymeric Micelles Possessing Poly(N-isopropylacrylamide)-Based Outer Coronas. Mol. Pharm. 2010, 7, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Hiruta, Y.; Shimamura, M.; Matsuura, M.; Maekawa, Y.; Funatsu, T.; Suzuki, Y.; Ayano, E.; Okano, T.; Kanazawa, H. Temperature-Responsive Fluorescence Polymer Probes with Accurate Thermally Controlled Cellular Uptakes. ACS Macro Lett. 2014, 3, 281–285. [Google Scholar] [CrossRef]
- Hiruta, Y.; Nagumo, Y.; Suzuki, Y.; Funatsu, T.; Ishikawa, Y.; Kanazawa, H. The effects of anionic electrolytes and human serum albumin on the LCST of poly(N-isopropylacrylamide)-based temperature-responsive copolymers. Colloids Surf. B 2015, 132, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, H.; Kashiwase, Y.; Yamamoto, K.; Matsushima, Y.; Kikuchi, A.; Sakurai, Y.; Okano, T. Temperature-Responsive Liquid Chromatography. 2. Effects of Hydrophobic Groups in N-Isopropylacrylamide Copolymer-Modified Silica. Anal. Chem. 1997, 69, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Kumazaki, M.; Kanazawa, H.; Kobayashi, J.; Kikuchi, A.; Akiyama, Y.; Annaka, M.; Okano, T. Thermoresponsive Polymer Brush Surfaces with Hydrophobic Groups for All-Aqueous Chromatography. ACS Appl. Mater. Interfaces 2010, 2, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Sawaji, Y.; Sato, T.; Takeuchi, A.; Hirata, M.; Ito, A. Anti-angiogenic action of hyperthermia by suppressing gene expression and production of tumour-derived vascular endothelial growth factor in vivo and in vitro. Br. J. Cancer 2002, 86, 1597–1603. [Google Scholar] [CrossRef] [Green Version]
- Verwey, E.J.W. Theory of the Stability of Lyophobic Colloids. J. Phys. Colloid Chem. 1947, 51, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Marra, J.; Israelachvili, J. Direct measurements of forces between phosphatidylcholine and phosphatidylethanolamine bilayers in aqueous electrolyte solutions. Biochemistry 1985, 24, 4608–4618. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeong, J.H.; Lee, S.H.; Kim, S.W.; Park, T.G. Local and systemic delivery of VEGF siRNA using polyelectrolyte complex micelles for effective treatment of cancer. J. Control. Release 2008, 129, 107–116. [Google Scholar] [CrossRef]
- Christie, R.J.; Matsumoto, Y.; Miyata, K.; Nomoto, T.; Fukushima, S.; Osada, K.; Halnaut, J.; Pittella, F.; Kim, H.J.; Nishiyama, N.; et al. Targeted Polymeric Micelles for siRNA Treatment of Experimental Cancer by Intravenous Injection. ACS Nano 2012, 6, 5174–5189. [Google Scholar] [CrossRef]










| Polymer | Molecular Weight | LCST (°C) d | |||
|---|---|---|---|---|---|
| GPC | 1H NMR b | Titration c | |||
| Mn a | Mw a | ||||
| P(NIPAAm-co-DMAPAAm) | 17,000 | 4.4 | 11,000 | 41.9 | |
| P(NIPAAm-co-DMAAm) | 15,000 | 3.5 | 13,500 | 41.7 | |
| Modified Polymer | Sample a | Diameter b (nm) | PDI b | Zeta Potential d (mV) |
|---|---|---|---|---|
| P(NIPAAm-co-DMAPAAm) | siRNA (−) | 166.6 ± 1.5 | 0.14 | 43.05 ± 1.08 |
| charge ratio (+/−) = 5 | 168.3 ± 1.0 | 0.08 | 20.95 ± 0.13 | |
| charge ratio (+/−) = 10 | 171.4 ± 7.9 | 0.07 | 27.78 ± 1.64 | |
| charge ratio (+/−) = 20 | 169.1 ± 7.3 | 0.09 | 35.24 ± 0.36 | |
| P(NIPAAm-co-DMAAm) | siRNA (−) | 164.2 ± 7.3 | 0.19 | 52.32 ± 1.39 |
| charge ratio (+/−) = 5 | 170.5 ± 2.4 | 0.12 | 23.28 ± 0.69 | |
| charge ratio (+/−) = 10 | 158.1 ± 6.9 | 0.12 | 35.22 ± 0.72 | |
| charge ratio (+/−) = 20 | 150.3 ± 0.4 | 0.13 | 38.79 ± 0.88 | |
| Non-modified | siRNA (−) | 135.8 ± 0.7 | 0.24 | 62.06 ± 0.35 |
| charge ratio (+/−) = 5 | N.D. c | N.D. c | 29.16 ± 5.20 | |
| charge ratio (+/−) = 10 | 47.45 ± 0.57 | |||
| charge ratio (+/−) = 20 | 50.67 ± 1.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagase, K.; Hasegawa, M.; Ayano, E.; Maitani, Y.; Kanazawa, H. Effect of Polymer Phase Transition Behavior on Temperature-Responsive Polymer-Modified Liposomes for siRNA Transfection. Int. J. Mol. Sci. 2019, 20, 430. https://doi.org/10.3390/ijms20020430
Nagase K, Hasegawa M, Ayano E, Maitani Y, Kanazawa H. Effect of Polymer Phase Transition Behavior on Temperature-Responsive Polymer-Modified Liposomes for siRNA Transfection. International Journal of Molecular Sciences. 2019; 20(2):430. https://doi.org/10.3390/ijms20020430
Chicago/Turabian StyleNagase, Kenichi, Momoko Hasegawa, Eri Ayano, Yoshie Maitani, and Hideko Kanazawa. 2019. "Effect of Polymer Phase Transition Behavior on Temperature-Responsive Polymer-Modified Liposomes for siRNA Transfection" International Journal of Molecular Sciences 20, no. 2: 430. https://doi.org/10.3390/ijms20020430
APA StyleNagase, K., Hasegawa, M., Ayano, E., Maitani, Y., & Kanazawa, H. (2019). Effect of Polymer Phase Transition Behavior on Temperature-Responsive Polymer-Modified Liposomes for siRNA Transfection. International Journal of Molecular Sciences, 20(2), 430. https://doi.org/10.3390/ijms20020430