Deep Eutectic Solvents as New Reaction Media to Produce Alkyl-Glycosides Using Alpha-Amylase from Thermotoga maritima
Abstract
:1. Introduction
2. Results
2.1. Screening of DES for Application in α-amylase Catalyzed Reactions and Effect of Water Content on Enzyme Activity and Stability
2.2. Screening for Transfer to Alcohols (Alcoholysis) in Selected DES
2.3. Exploration of Alcoholysis Activity in Choline Chloride:Urea (1:2):Water DES Using Starch as Glucosyl Donor
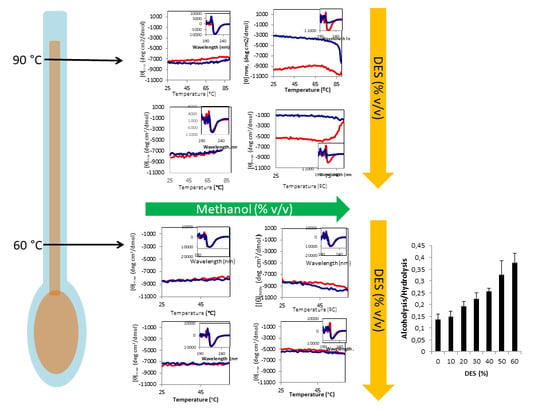
2.4. Stability of Amy A in Mixtures of DES-Methanol
2.5. Activity of Amy A at 60 °C
2.6. Alcoholysis Yield by Amy A at 60 °C
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Analytical Procedures
4.3. Synthesis of Deep Eutectic Solvents (DES)
4.4. Screening of Deep Eutectic Solvents for Application in α-amylase Catalyzed Reactions
4.5. Stability of Amy A in the Different Deep Eutectic Solvents
4.6. Screening for Alcoholytic Activity in Selected DES
4.7. Product Quantification by HPLC
4.8. Hydrolysis Activity Assay
4.9. Alcoholysis Reactions with Methanol and Starch in Choline Chloride:Urea 1:2 DES as co Solvent
4.10. Hydrolysis Quantification at the End Point of Reaction
4.11. Alcoholysis Reaction Measurement
4.12. Alcoholysis Yield and Alcoholysis/Hydrolysis Ratio
4.13. Structural Characterization of Amy A in Choline Chloride:Urea 1:2 DES as co Solvent
4.14. Thermal Stability of Amy A in Choline Chloride:Urea 1:2 DES as co Solvent
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larsson, J.; Svensson, D.; Adlercreutz, P. α-Amylase-catalysed synthesis of alkyl glycosides. J. Mol. Catal. B Enzym. 2005, 37, 84–87. [Google Scholar] [CrossRef]
- Matsumura, S.; Imai, K.; Yoshikawa, S.; Kawada, K.; Uchibori, T. Surface activities, biodegradability and antimicrobial properties of n-alkyl glucosides, mannosides and galactosides. J. Am. Oil Chem. Soc. 1990, 67, 996–1001. [Google Scholar] [CrossRef]
- Rather, M.Y.; Mishra, S. β-glycosidases: An alternative enzyme based method for synthesis of alkyl-glycosides. Sustain. Chem. Process. 2013, 1, 7. [Google Scholar] [CrossRef]
- Iglauer, S.; Wu, Y.; Shuler, P.; Tang, Y.; Goddard, W.A. Analysis of the influence of alkyl polyglycoside surfactant and cosolvent structure on interfacial tension in aqueous formulations versus n-octane. Tenside Surfactants Deterg. 2010, 47, 87–97. [Google Scholar] [CrossRef]
- Ismail, A.; Soultani, S.; Ghoul, M. Enzymatic-catalyzed synthesis of alkylglycosides in monophasic and biphasic systems. I. The transglycosylation reaction. J. Biotech. 1999, 69, 135–143. [Google Scholar] [CrossRef]
- Fink, M.J.; Syrén, P.-O. Redesign of water networks for efficient biocatalysis. Curr. Opin. Chem. Biol. 2017, 37, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Farnberger, J.E.; Kroutil, W. The industrial age of biocatalytic transamination. Eur. J. Org. Chem. 2015, 2015, 6965–6982. [Google Scholar] [CrossRef]
- Gotor, V. Biocatalysis applied to the preparation of pharmaceuticals. Org. Proc. Res. Dev. 2002, 6, 420–426. [Google Scholar] [CrossRef]
- Kulishova, L.M.; Zharkov, D.O. Solid/gas biocatalysis. Biochemistry 2017, 82, 95–105. [Google Scholar] [CrossRef]
- Polakovič, M.; Švitel, J.; Bučko, M.; Filip, J.; Neděla, V.; Ansorge-Schumacher, M.B.; Gemeiner, P. Progress in biocatalysis with immobilized viable whole cells: Systems development, reaction engineering and applications. Biotechnol. Lett. 2017, 39, 667–683. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Pereira, P.C. Biocatalysis engineering: The big picture. Chem. Soc. Rev. 2017, 46, 2678–2691. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Zaks, A. Industrial biocatalysis. Curr. Opin. Chem. Biol. 2001, 5, 130–136. [Google Scholar] [CrossRef]
- Panintrarux, C.; Adachi, S.; Matsuno, R. β-glucosidase-catalyzed condensation of glucose with 2-alcohols in buffer-saturated alcohols. Biotechnol. Lett. 1997, 19, 899–902. [Google Scholar] [CrossRef]
- Kamiyama, Y.; Yasui, T. Enzymatic synthesis of alkyl β-xylosides from xylobiose by application of the transxylosyl reaction of aspergillus niger β-xylosidase AU—Shinoyama, Hirofumi. Agric. Biol. Chem. 1988, 52, 2197–2202. [Google Scholar] [CrossRef]
- Damian-Almazo, J.Y.; Moreno, A.; Lopez-Munguia, A.; Soberon, X.; Gonzalez-Munoz, F.; Saab-Rincon, G. Enhancement of the alcoholytic activity of α-amylase Amy A from Thermotoga maritima MSB8 (DSM 3109) by site directed mutagenesis. Appl. Environ. Microbiol. 2008, 74, 5168–5177. [Google Scholar] [CrossRef]
- Saab-Rincon, G.; del-Rio, G.; Santamaria, R.I.; Lopez-Munguia, A.; Soberon, X. Introducing transglycosylation activity in a liquefying α-amylase. FEBS Lett. 1999, 453, 100–106. [Google Scholar] [CrossRef]
- Santamaria, R.I.; Del, R.G.; Saab, G.; Rodriguez, M.E.; Soberon, X.; Lopez, M.A. Alcoholysis reactions from starch with alpha-amylases. FEBS Lett. 1999, 452, 346–350. [Google Scholar] [CrossRef]
- Vihinen, M.; Ollikka, P.; Niskanen, J.; Meyer, P.; Suominen, I.; Karp, M.; Holm, L.; Knowles, J.; Mantsala, P. Site-directed mutagenesis of a thermostable alpha-amylase from Bacillus stearothermophilus: Putative role of three conserved residues. J. Biochem. 1990, 107, 267–272. [Google Scholar] [CrossRef]
- Moreno, A.; Damian-Almazo, J.Y.; Miranda, A.; Saab-Rincon, G.; Gonzalez, F.; Lopez-Munguia, A. Transglycosylation reactions of Thermotoga maritima α-amylase. Enzym. Microb. Tech. 2010, 46, 331–337. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Baréa, B.; Piombo, G.; Dubreucq, E.; Villeneuve, P. Evaluation of deep eutectic solvents as new media for Candida antarctica B lipase catalyzed reactions. Process Biochem. 2012, 47, 2081–2089. [Google Scholar] [CrossRef]
- Juneidi, I.; Hayyan, M.; Hashim, M.A.; Hayyan, A. Pure and aqueous deep eutectic solvents for a lipase-catalysed hydrolysis reaction. Biochem. Eng. J. 2017, 117, 129–138. [Google Scholar] [CrossRef]
- Khodaverdian, S.; Dabirmanesh, B.; Heydari, A.; Dashtban-moghadam, E.; Khajeh, K.; Ghazi, F. Activity, stability and structure of laccase in betaine based natural deep eutectic solvents. Int. J. Biol. Macromol. 2018, 107, 2574–2579. [Google Scholar] [CrossRef] [PubMed]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Larsson, K.M.; Kirk, O. One biocatalyst–many applications: The use of Candida Antarctica B-lipase in organic synthesis. Biocatal. Biotransform. 1998, 16, 183–204. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef]
- Bell, G.; Halling, P.J.; Moore, B.D.; Partridge, J.; Rees, D.G. Biocatalyst behaviour in low-water systems. Trends Biotechnol. 1995, 13, 468–473. [Google Scholar] [CrossRef]
- Chahid, Z.; Montet, D.; Pina, M.; Graille, J. Effect of water activity on enzymatic synthesis of alkylglycosides. Biotechnol. Lett. 1992, 14, 281–284. [Google Scholar] [CrossRef]
- Klibanov, A.M. Enzymatic catalysis in anhydrous organic solvents. Trends Biochem. Sci. 1989, 14, 141–144. [Google Scholar] [CrossRef]
- Montiel, C.; Bustos-Jaimes, I.; Bárzana, E. Enzyme-catalyzed synthesis of heptyl-β-glycosides: Effect of water coalescence at high temperature. Bioresour. Technol. 2013, 144, 135–140. [Google Scholar] [CrossRef]
- Vulfson, E.N.; Patel, R.; Law, B.A. Alkyl-β-glucoside synthesis in a water-organic two-phase system. Biotechnol. Lett. 1990, 12, 397–402. [Google Scholar] [CrossRef]
- Qin, H.; Hu, X.; Wang, J.; Cheng, H.; Chen, L.; Qi, Z. Overview of acidic deep eutectic solvents on synthesis, properties and applications. Green Energy Environ. 2019. [Google Scholar] [CrossRef]
- Skulcova, A.; Russ, A.; Jablonsky, M.; Sima, J. The pH behavior of seventeen deep eutectic solvents. Bioresources 2018, 13, 5042–5051. [Google Scholar] [CrossRef]
- Hernandez-Meza, J.M.; Sampedro, J.G. Trehalose mediated inhibition of lactate dehydrogenase from rabbit muscle. The application of Kramers’ theory in enzyme catalysis. J. Phys. Chem. B 2018, 122, 4309–4317. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, J.G.; Uribe, S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition. The role of viscosity. Mol. Cell Biochem. 2004, 256–257, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Uribe, S.; Sampedro, J.G. Measuring solution viscosity and its effect on enzyme activity. Biol. Proced. Online 2003, 5, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, M.C.; Ferrer, M.L.; Yuste, L.; Rojo, F.; del Monte, F. Bacteria incorporation in deep-eutectic solvents through freeze-drying. Angew. Chem. Int. Ed. Engl. 2010, 49, 2158–2162. [Google Scholar] [CrossRef]
- Summer, J.B.; Howell, S.F. A method for determination of saccharase activity. J. Biol. Chem. 1935, 108, 51–54. [Google Scholar]








| Condition | KM (mg/mL) | Vmax (µmol/mg *min) |
|---|---|---|
| No DES | 2.8 ± 0.5 | 196 ± 10 |
| 60% DES | 5.7± 1.7 | 27 ± 4 |
| 60% DES 10% MeOH | 1.7± 0.6 | 3.6 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Molina, A.; Xolalpa, W.; Strompen, S.; Arreola-Barroso, R.; Olvera, L.; López-Munguía, A.; Castillo, E.; Saab-Rincon, G. Deep Eutectic Solvents as New Reaction Media to Produce Alkyl-Glycosides Using Alpha-Amylase from Thermotoga maritima. Int. J. Mol. Sci. 2019, 20, 5439. https://doi.org/10.3390/ijms20215439
Miranda-Molina A, Xolalpa W, Strompen S, Arreola-Barroso R, Olvera L, López-Munguía A, Castillo E, Saab-Rincon G. Deep Eutectic Solvents as New Reaction Media to Produce Alkyl-Glycosides Using Alpha-Amylase from Thermotoga maritima. International Journal of Molecular Sciences. 2019; 20(21):5439. https://doi.org/10.3390/ijms20215439
Chicago/Turabian StyleMiranda-Molina, Alfonso, Wendy Xolalpa, Simon Strompen, Rodrigo Arreola-Barroso, Leticia Olvera, Agustín López-Munguía, Edmundo Castillo, and Gloria Saab-Rincon. 2019. "Deep Eutectic Solvents as New Reaction Media to Produce Alkyl-Glycosides Using Alpha-Amylase from Thermotoga maritima" International Journal of Molecular Sciences 20, no. 21: 5439. https://doi.org/10.3390/ijms20215439
APA StyleMiranda-Molina, A., Xolalpa, W., Strompen, S., Arreola-Barroso, R., Olvera, L., López-Munguía, A., Castillo, E., & Saab-Rincon, G. (2019). Deep Eutectic Solvents as New Reaction Media to Produce Alkyl-Glycosides Using Alpha-Amylase from Thermotoga maritima. International Journal of Molecular Sciences, 20(21), 5439. https://doi.org/10.3390/ijms20215439