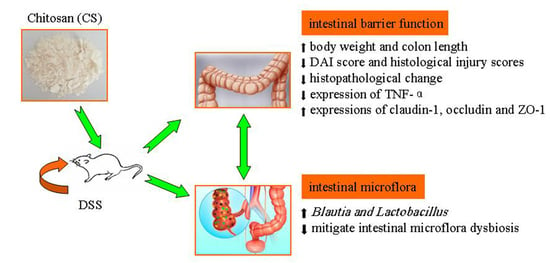
Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora
Abstract
:1. Introduction
2. Results
2.1. Effects of CS on Body Weight and DAI in DSS-Induced UC Mice
2.2. Effects of CS on Colon Length and Histopathology in DSS-Induced UC Mice
2.3. Effects of CS on Expressions of TNF-α, Claudin-1, Occludin, and ZO-1
2.4. PCR-DGGE Analysis
3. Discussions
4. Materials and Methods
4.1. Material and Reagents
4.2. Animals and Experimental Design
4.3. Evaluation of Disease Activity Index
4.4. Colon Histopathology
4.5. Western Blotting Assay
4.6. Polymerse Chain Rection (PCR)-Denaturing Gradient Gel Electrophoresis (DGGE) Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adams, S.M.; Bornemann, P.H. Ulcerative colitis. Am. Fam. Physician 2013, 87, 699–705. [Google Scholar] [PubMed]
- Zhang, Z.L.; Fan, H.Y.; Yang, M.Y.; Zhang, Z.K.; Liu, K. Therapeutic effect of a hydroxynaphthoquinone fraction on dextran sulfate sodium-induced ulcerative colit. World J. Gastroenterol. 2014, 20, 15310. [Google Scholar] [CrossRef] [PubMed]
- Actis, G.C.; Pellicano, R.; Rosina, F. Inflammatory bowel disease: Traditional knowledge holds the seeds for the future. World J. Gastrointest. Pharmacol. Ther. 2015, 6, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cai, Y.Y.; Yan, Z.X. Brain-derived neurotrophic factor preserves intestinal mucosal barrier function and alters gut microbiota in mice. Kaohsiung J. Med. Sci. 2018, 34, 134–141. [Google Scholar] [CrossRef]
- Yang, F.J.; Wang, A.N.; Zeng, X.F.; Hou, C.L.; Liu, H.; Qiao, S.Y. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015, 15, 32–42. [Google Scholar] [CrossRef]
- Ren, X.; Zhu, Y.; Gamallat, Y.; Ma, S.; Chiwala, G.; Meyiah, A.; Xin, Y. E. coli O124 K72 alters the intestinal barrier and the tight junctions proteins of guinea pig intestine. Biomed. Pharmacother. 2017, 94, 468–473. [Google Scholar] [CrossRef]
- Vargas Robles, H.; Castro Ochoa, K.F.; Nava, P.; Silva Olivares, A.; Shibayama, M.; Schnoor, M. Analyzing beneficial effects of nutritional supplements on intestinal epithelial barrier functions during experimental colitis. J. Vis. Exp. 2017, 2017, e55095. [Google Scholar] [CrossRef]
- Lamsal, B.P. Production, health aspects and potential food uses of dairy prebiotic galactooligosaccharides. J. Sci. Food Agric. 2012, 92, 2020–2028. [Google Scholar] [CrossRef]
- Peshev, D.; Ende, W.V. Fructans: Prebiotics and immunomodulators. J. Funct. Foods 2014, 8, 348–357. [Google Scholar] [CrossRef]
- Diaz, Y.M.; Laverde, G.V.; Gamba, L.R.; Wandurraga, H.M.; Ferrom, C.A. Biofilm inhibition activity of compounds isolated from two Eunicea species collected at the Caribbean Sea. Rev. Bras. Farmacogn. 2015, 25, 605–611. [Google Scholar] [CrossRef]
- Rubini, D.; Farisa Banu, S.; Veda Hari, B.N.; Ramya Devi, D.; Gowrishankar, S.; Karutha Pandian, S.; Nithyanand, P. Chitosan extracted from marine biowaste mitigates staphyloxanthin production and biofilms of Methicillin-resistant Staphylococcus aureus. Food Chem. Toxicol. 2018, 118, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Wang, Y.; Liu, G.; He, J.; Qiu, W.; Hu, X.; Feng, Z.; Ran, M.; Nyachoti, C.M.; Kim, S.W.; et al. Effects of chitosan on intestinal inflammation in weaned pigs challenged by enterotoxigenic Escherichia coli. PLoS ONE 2014, 9, e104192. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Jo, S.H.; Ha, K.S.; Kim, S.C.; Kim, Y.C.; Apostolidis, E.; Kwon, Y.I. Effect of long-term supplementation of low molecular weight chitosan oligosaccharide (GO2KA1) on fasting blood glucose and HbA1c in db/db mice model and elucidation of mechanism of action. BMC Complement Altern. Medi. 2014, 14, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, J.; Gui, S. Orally targeted galactosylated chitosan poly(lactic-co-glycolic acid) nanoparticles loaded with TNF-α siRNA provide a novel strategy for the experimental treatment of ulcerative colitis. Eur. J. Pharm. Sci. 2018, 125, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tam, M.; Samaei, S.; Lerouge, S.; Barralet, J.; Stevenson, M.M.; Cerruti, M. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomaterialia. 2017, 48, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.K.; Wang, Y.; Kong, M.; Li, X.L. Prebiotic-like effects of water soluble chitosan on the intestinal microflora in mice. Int. J. Food Eng. 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Tan, Y.; Zheng, C. Effects of alpinetin on intestinal barrier function, inflammation and oxidative stress in dextran sulfate sodium-induced ulcerative colitis mice. Am. J. Med. Sci. 2018, 55, 377–386. [Google Scholar] [CrossRef]
- Azzam, M.M.; Zou, X.T.; Dong, X.Y.; Xie, P. Effect of supplemental L-threonine on mucin 2 gene expression and intestine mucosal immune and digestive enzymes activities of laying hens in environments with high temperature and humidity. Poult. Sci. 2011, 90, 2251–2256. [Google Scholar] [CrossRef]
- Choi, S.; Woo, J.K.; Jang, Y.S.; Kang, J.H.; Jang, J.E.; Yi, T.H.; Park, S.Y.; Kim, S.Y.; Yoon, Y.S.; Oh, S.H. Fermented Pueraria lobata extract ameliorates dextran sulfate sodium-induced colitis by reducing pro-inflammatory cytokines and recovering intestinal barrier function. Lab. Anim. Res. 2016, 32, 151–159. [Google Scholar] [CrossRef]
- Li, H.H.; Li, Y.P.; Zhu, Q.; Qiao, J.Y.; Wang, W.J. Dietary supplementation with Clostridium butyricum helps to improve the intestinal barrier function of weaned piglets challenged with enterotoxigenic Escherichia coli K88. J. Appl. Microbiol. 2018, 125, 964–975. [Google Scholar] [CrossRef]
- He, L.X.; Wang, J.B.; Sun, B.; Zhao, J.; Li, L.; Xu, T.; Li, H.; Sun, J.Q.; Ren, J.; Liu, R.; et al. Suppression of TNF-α and free radicals reduces systematic inflammatory and metabolic disorders: Radioprotective effects of ginseng oligopeptides on intestinal barrier function and antioxidant defense. J. Nutr. Biochem. 2017, 40, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Rosenstiel, P.; Fantini, M.; Bräutigam, K.; Kühbacher, T.; Waetzig, G.H.; Seegert, D.; Schreiber, S. TNF-α and IFN-γ regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology 2003, 124, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ouyang, Q. Effects of Bawei XileiSan on mice with oxazolone-induced colitis and the mechanisms. J. Chin. Integr. Med. 2010, 8, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Löwenberg, M.; D’Haens, G. Novel targets for inflammatory bowel disease therapeutics. Curr. Gastroenterol. Rep. 2013, 15, 311–316. [Google Scholar] [CrossRef]
- Blackwood, B.P.; Yuan, C.Y.; Wood, D.R.; Nicolas, J.D.; Grothaus, J.S.; Hunter, C.J. Probiotic Lactobacillus species strengthen intestinal barrier function and tight junction integrity in experimental necrotizing enterocolitis. J. Probiotics Health. 2017, 5, 59–78. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, T.; Wang, X.; Kuang, S.; Xiao, Y.; Lu, W.W.; Bi, D.R. Probiotic mixture ameliorates heat stress of laying hens by enhancing intestinal barrier function and improving gut microbiota. Ital. J. Anim. Sci. 2017, 16, 292–300. [Google Scholar] [CrossRef]
- Sun, X.; Du, M.; Navarre, D.A.; Zhu, M.J. Purple potato extract promotes intestinal epithelial differentiation and barrier function by activating AMP-activated protein kinase. Mol. Nutr. Food Res. 2018, 62, 1700536. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, M.S.; Bae, J.W. Blautia faecis sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2013, 63, 599–603. [Google Scholar] [CrossRef]
- Hamajima, H.; Matsunaga, H.; Fujikawa, A.; Sato, T.; Mitsutake, S.; Yanagita, T.; Nagao, K.; Nakayama, J.; Kitagaki, H. Japanese traditional dietary fungus koji Aspergillus oryzae functions as a prebiotic for Blautia coccoides through glycosylceramide: Japanese dietary fungus koji is a new prebiotic. Springerplus 2016, 5, 1321–1330. [Google Scholar] [CrossRef]
- Jiang, D.; Kang, A.; Yao, W.; Lou, J.; Zhang, Q.; Bao, B.; Cao, Y.; Yu, S.; Guo, S.; Zhang, Y.; et al. Euphorbia kansui fry-baked with vinegar modulates gut microbiota and reduces intestinal toxicity in rats. J. Ethnopharmacol. 2018, 226, 26–35. [Google Scholar] [CrossRef]
- Lakhdari, O.; Tap, J.; Béguet-Crespel, F.; Le Roux, K.; de Wouters, T.; Cultrone, A.; Nepelska, M.; Lefèvre, F.; Doré, J.; Blottière, H.M. Identification of NF-κB modulation capabilities within human intestinal commensal bacteria. J. Biomed. Biotechnol. 2011, 2011, 282356. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, G.; Mahajan, U.B.; Goyal, S.N.; Ojha, S.; Patil, C.R.; Subramanya, S.B. Protective effect of Lagerstroemia speciosa against dextran sulfate sodium induced ulcerative colitis in C57BL/6 mice. Am. J. Transl. Res. 2017, 9, 1792–1800. [Google Scholar]
- Yan, H.; Wang, H.; Zhang, X.; Li, X.; Yu, J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. Int. J. Clin. Exp. Med. 2015, 8, 20245–20253. [Google Scholar]
- Li, X.L.; Zhang, C.L.; Li, W.L.; Wu, D.C.; Liu, J.J.; Tang, L.; Xin, Y. In vivo effects on the intestinal microflora of Physalis alkekengi var. francheti extracts. Fitoterapia 2013, 87, 43–48. [Google Scholar] [CrossRef]




| Selected Band | Most Similar Sequence Relative (GenBank Accession Number) | Bacteria Genus | Identity (%) |
|---|---|---|---|
| a | Parabacteroides distasonis (NZ 009615.1) | Parabacteroides | 83 |
| b | Parabacteroides sp. (NC CP015402.2) | 91 | |
| c | Blautia sp. (NZ CP015405.2) | Blautia | 97 |
| d | Lactobacillus johnsonii (NZ 022909.1) | Lactobacillus | 92 |
| e | Lactobacillus ruminis (NZ 015975.1) | 91 | |
| f | Prevotella intermedia (NZ CP019301.1) | Prevotella | 87 |
| g | Prevotella dentalis (NZ 019968.1) | 89 |
| Group | S | H′ | E |
|---|---|---|---|
| D11-Group1 | 20.50 ± 1.29 | 3.0679 ± 0.1258 | 1.0165 ± 0.0439 |
| D11-Group2 | 18.50 ± 0.58 * | 2.7446 ± 0.0469 ** | 0.9410 ± 0.0259 * |
| D11-Group3 | 20.25 ± 0.96 | 2.9627 ± 0.0473 | 0.9852 ± 0.0111 |
| D11-Group4 | 18.75 ± 0.50 * | 2.9097 ± 0.0270 | 0.9929 ± 0.0218 |
| D15-Group1 | 19.75 ± 0.96 | 2.9585 ± 0.0480 | 0.9922 ± 0.0226 |
| D15-Group2 | 20.25 ± 0.50 | 2.8867 ± 0.0244 * | 0.9597 ± 0.0145 * |
| D15-Group3 | 19.25 ± 0.96 | 2.9241 ± 0.0270 | 0.9889 ± 0.0267 |
| D15-Group4 | 20.00 ± 0.82 | 2.9269 ± 0.0409 | 0.9775 ± 0.0279 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. Int. J. Mol. Sci. 2019, 20, 5751. https://doi.org/10.3390/ijms20225751
Wang J, Zhang C, Guo C, Li X. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. International Journal of Molecular Sciences. 2019; 20(22):5751. https://doi.org/10.3390/ijms20225751
Chicago/Turabian StyleWang, Jia, Cuili Zhang, Chunmei Guo, and Xinli Li. 2019. "Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora" International Journal of Molecular Sciences 20, no. 22: 5751. https://doi.org/10.3390/ijms20225751
APA StyleWang, J., Zhang, C., Guo, C., & Li, X. (2019). Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. International Journal of Molecular Sciences, 20(22), 5751. https://doi.org/10.3390/ijms20225751