Radiochemical Approaches to Imaging Bacterial Infections: Intracellular versus Extracellular Targets
Abstract
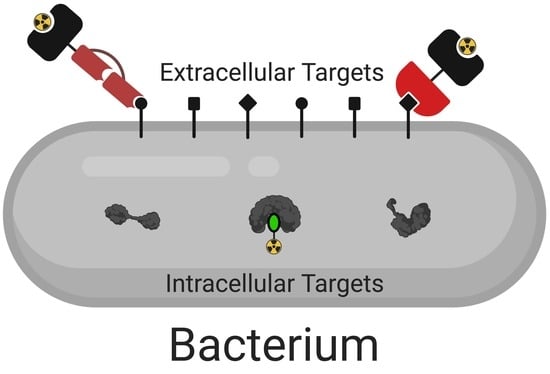
:1. Introduction
2. Radionuclide Choice: Chelation vs. Covalent Attachment
2.1. Chelation of Radionuclide
2.2. Covalent Attachment of Radionuclide
3. Intracellular Targets
3.1. Inhibitors of Peptidoglycan Cross-Linking–β-lactams
3.2. Inhibitors of Protein Synthesis–Macrolide Antibiotics
3.3. Inhibitors of DNA Synthesis–Fluoroquinolones
3.4. Inhibitors of Folic Acid Synthesis–Sulfonamides and Trimethoprim
3.5. Metabolic Tracers
4. Extracellular Targets
4.1. Antimicrobial Peptides
4.2. Antibodies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| PCR | Polymerase chain reaction |
| MRI | Magnetic resonance imaging |
| US | Ultrasound |
| CT | Computed tomography |
| PET | Positron emission tomography |
| SPECT | Single-photon emission computed tomography |
| DOTATATE | DOTA-octreotide |
| FDG | Fluorodeoxyglucose |
| PBP | Penicillin-binding protein |
| T/NT | Target-to-nontarget ratio |
| MIC | Minimum inhibitory concentration |
| PABA | Para-aminobenzoic acid |
| SMX | Sulfamethoxazole |
| TMP | Trimethoprim |
| DHFR | Dihydrofolate reductase |
| AMP | Antimicrobial Peptide |
| LPS | Lipopolysaccharide |
| LTA | Lipoteichoic Acid |
| UBI | Ubiquicidin |
| t½ | Half-life |
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Scott, R.D. The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2009.
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Palestro, C.J. Radionuclide imaging of infection: In search of the grail. J. Nucl. Med. 2009, 50, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.; Roca, M.; Martin-Comin, J. Labeling of antibiotics for infection diagnosis. Q. J. Nucl. Med. Mol. Imaging 2006, 50, 147–152. [Google Scholar] [PubMed]
- Ordonez, A.A.; Sellmyer, M.A.; Gowrishankar, G.; Ruiz-Bedoya, C.A.; Tucker, E.W.; Palestro, C.J.; Hammoud, D.A.; Jain, S.K. Molecular imaging of bacterial infections: Overcoming the barriers to clinical translation. Sci. Transl. Med. 2019, 11, eaax8251. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K. The promise of molecular imaging in the study and treatment of infectious diseases. Mol. Imaging Biol. 2017, 19, 341–347. [Google Scholar] [CrossRef]
- Welling, M.M.; Hensbergen, A.W.; Bunschoten, A.; Velders, A.H.; Roestenberg, M.; van Leeuwen, F.W.B. An update on radiotracer development for molecular imaging of bacterial infections. Clin. Transl. Imaging 2019, 7, 105–124. [Google Scholar] [CrossRef]
- Auletta, S.; Galli, F.; Lauri, C.; Martinelli, D.; Santino, I.; Signore, A. Imaging bacteria with radiolabelled quinolones, cephalosporins and siderophores for imaging infection: A systematic review. Clin. Transl. Imaging 2016, 4, 229–252. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Imran, M.B.; Nadeem, M.A.; Shahid, A. Antimicrobial peptides as infection imaging agents: Better than radiolabeled antibiotics. Int. J. Pept. 2012, 2012, 1–19. [Google Scholar] [CrossRef]
- Heuker, M.; Gomes, A.; Dijl, J.M.; Dam, G.M.; Friedrich, A.W.; Sinha, B.; Oosten, M. Preclinical studies and prospective clinical applications for bacteria-targeted imaging: The future Is bright. Clin. Transl. Imaging 2016, 4, 253–264. [Google Scholar] [CrossRef]
- Sellmyer, M.A.; Lee, I.; Hou, C.; Weng, C.-C.; Li, S.; Lieberman, B.P.; Zeng, C.; Mankoff, D.A.; Mach, R.H. Bacterial Infection Imaging with [18F]Fluoropropyl-Trimethoprim. Proc. Natl. Acad. Sci. USA 2017, 114, 8372–8377. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, G.; Hardy, J.; Wardak, M.; Namavari, M.; Reeves, R.E.; Neofytou, E.; Srinivasan, A.; Wu, J.C.; Contag, C.H.; Gambhir, S.S. Specific imaging of bacterial infection using 6″-18F-fluoromaltotriose: A second-generation PET tracer targeting the maltodextrin transporter in bacteria. J. Nucl. Med. 2017, 58, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, A.A.; Weinstein, E.A.; Bambarger, L.E.; Saini, V.; Chang, Y.S.; DeMarco, V.P.; Klunk, M.H.; Urbanowski, M.E.; Moulton, K.L.; Murawski, A.M.; et al. A systematic approach for developing bacteria-specific imaging tracers. J. Nucl. Med. 2017, 58, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, S.K.; Qadir, M.A.; Shabnam, S.; Javed, M. 99mTc-amoxicillin: A novel radiopharmaceutical for infection imaging. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Demiroglu, H.; Topal, G.; Parlak, Y.; Gumuser, F.G.; Turkoz, E.U.; Tekin, V.; Ates, B.; Unak, P.; Avcibasi, U. Radiosynthesis and biodistribution of 99mTc-trimethoprim: A novel radiolabeled antibiotic for bacterial infection imaging using experimental animals. Kafkas Univ. Vet. Fak Derg. 2018, 24, 393–400. [Google Scholar]
- Weinstein, E.A.; Ordonez, A.A.; DeMarco, V.P.; Murawski, A.M.; Pokkali, S.; MacDonald, E.M.; Klunk, M.; Mease, R.C.; Pomper, M.G.; Jain, S.K. Imaging enterobacteriaceae infection in vivo with F-18-fluorodeoxysorbitol positron emission tomography. Sci. Transl. Med. 2014, 6, 259ra146. [Google Scholar] [CrossRef]
- Lewis, J.S.; Windhorst, A.D.; Zeglis, B.M. Radiopharmaceutical Chemistry; Springer International Publishing: Cham, Switzerland, 2018; Volume 37, pp. 627–633. [Google Scholar]
- Radford, L.L.; Lapi, S.E. Methods for the production of radionuclides for medicine. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 63–83. [Google Scholar]
- Grupen, C. Introduction to Radiation Protection; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Holland, J.P. The Radiopharmaceutical chemistry of seldom-used radionuclides in nuclear medicine. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 425–446. [Google Scholar]
- Hofman, M.S.; Eddie Lau, W.F.; Hicks, R.J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation1. Radiographics 2015, 35, 500–516. [Google Scholar] [CrossRef]
- Rathmann, S.M.; Ahmad, Z.; Slikboer, S.; Bilton, H.A.; Snider, D.P.; Valliant, J.F. The Radiopharmaceutical Chemistry of Technetium-99m. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 311–333. [Google Scholar]
- Antoni, G. The Radiopharmaceutical chemistry of carbon-11: basic principles. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 207–220. [Google Scholar]
- Ermert, J.; Neumaier, B. The Radiopharmaceutical chemistry of fluorine-18: Nucleophilic fluorinations. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 273–283. [Google Scholar]
- Vaidyanathan, G.; Zalutsky, M.R. The radiopharmaceutical chemistry of the radioisotopes of iodine. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 391–408. [Google Scholar]
- Pichler, V.; Berroterán-Infante, N.; Ozenil, M.; Pfaff, S.; Philippe, C.; Wadsak, W. The Radiopharmaceutical Chemistry of Carbon-11: Tracers and Applications. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Radiopharmaceutical Chemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 221–236. [Google Scholar]
- Grassi, I.; Nanni, C.; Allegri, V.; Morigi, J.J.; Montini, G.C.; Castellucci, P.; Fanti, S. The Clinical Use of PET with (11)C-Acetate. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 33–47. [Google Scholar]
- Lewis, P.J.; Salama, A. Uptake of Fluorine-18-Fluorodeoxyglucose in Sarcoidosis. J. Nucl. Med. 1994, 35, 1647–1649. [Google Scholar]
- Jerusalem, G.; Beguin, Y.; Najjar, F.; Hustinx, R.; Fassotte, M.F.; Rigo, P.; Fillet, G. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) for the staging of low-grade non-hodgkin’s lymphoma (NHL). Ann. Oncol. 2001, 12, 825–830. [Google Scholar] [CrossRef]
- Mackie, G.C.; Shulkin, B.L.; Ribeiro, R.C.; Worden, F.P.; Gauger, P.G.; Mody, R.J.; Connolly, L.P.; Kunter, G.; Rodriguez-Galindo, C.; Wallis, J.W.; et al. Use of [18F]Fluorodeoxyglucose positron emission tomography in evaluating locally recurrent and metastatic adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Kubota, K.; Kubota, R.; Ido, T.; Tamahashi, N. High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J. Nucl. Med. 1995, 36, 1301–1306. [Google Scholar] [PubMed]
- Kong, K.-F.; Schneper, L.; Mathee, K. Beta-lactam antibiotics: From antibiosis to resistance and bacteriology. APMIS 2010, 118, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Hazari, P.P.; Rawat, H.; Singh, B.; Kalawat, T.C.; Sharma, S.; Babbar, A.K.; Mishra, A.K. Preliminary evaluation of technetium-99m-labeled ceftriaxone: Infection imaging agent for the clinical diagnosis of orthopedic infection. Int. J. Infect. Dis. 2013, 17, e263–e270. [Google Scholar] [CrossRef]
- Mostafa, M.; Motaleb, M.A.; Sakr, T.M. Labeling of ceftriaxone for infective inflammation imaging using 99mTc eluted from 99Mo/99mTc generator based on zirconium molybdate. Appl. Radiat. Isot. 2010, 68, 1959–1963. [Google Scholar] [CrossRef]
- Fazli, A.; Salouti, M.; Mazidi, M. 99mTc-ceftriaxone, as a targeting radiopharmaceutical for scintigraphic imaging of infectious foci due to staphylococcus aureus in mouse model. J. Radioanal. Nucl. Chem. 2013, 298, 1227–1233. [Google Scholar] [CrossRef]
- Abdel-Ghaney, I.Y.; Sanad, M.H. Synthesis of 99mTc-erythromycin complex as a model for infection sites imaging. Radiochemistry 2013, 55, 418–422. [Google Scholar] [CrossRef]
- Sanad, M.H. Labeling and biological evaluation of 99mTc-azithromycin for infective inflammation diagnosis. Radiochemistry 2013, 55, 539–544. [Google Scholar] [CrossRef]
- Borai, E.H.; Sanad, M.H.; Fouzy, A.S.M. Optimized chromatographic separation and biological evaluation of 99mTc-clarithromycin for infective inflammation diagnosis. Radiochemistry 2016, 58, 84–91. [Google Scholar] [CrossRef]
- Appelboom, T.; Emery, P.; Tant, L.; Dumarey, N.; Schoutens, A. Evaluation of Technetium-99m-Ciprofloxacin (Infecton) for Detecting Sites of Inflammation in Arthritis. Rheumatol. (Oxf.) 2003, 42, 1179–1182. [Google Scholar] [CrossRef]
- Gemmel, F.; De Winter, F.; Van Laere, K.; Vogelaers, D.; Uyttendaele, D.; Dierckx, R.A. 99mTc ciprofloxacin imaging for the diagnosis of infection in the postoperative spine. Nucl. Med. Commun. 2004, 25, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Sarda, L.; Crémieux, A.-C.; Lebellec, Y.; Meulemans, A.; Lebtahi, R.; Hayem, G.; Génin, R.; Delahaye, N.; Huten, D.; Le Guludec, D. Inability of 99mTc-ciprofloxacin scintigraphy to discriminate between septic and sterile osteoarticular diseases. J. Nucl. Med. 2003, 44, 920–926. [Google Scholar] [PubMed]
- De Winter, F.; Gemmel, F.; Van Laere, K.; De Winter, O.; Poffijn, B.; Dierckx, R.A.; Van de Wiele, C. 99mTc-ciprofloxacin planar and tomographic imaging for the diagnosis of infection in the postoperative spine: Experience in 48 patients. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Dumarey, N.; Blocklet, D.; Appelboom, T.; Tant, L.; Schoutens, A. Infecton is not specific for bacterial osteo-articular infective pathology. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 530–535. [Google Scholar] [CrossRef]
- Satpati, D.; Arjun, C.; Krishnamohan, R.; Samuel, G.; Banerjee, S. (68) Ga-labeled ciprofloxacin conjugates as radiotracers for targeting bacterial infection. Chem. Biol. Drug Des. 2016, 87, 680–686. [Google Scholar] [CrossRef]
- Peremans, K.; De Winter, F.; Janssens, L.; Dumont, F.; Van Bree, H.; Dierckx, R. An infected hip prosthesis in a dog diagnosed with a 99mTc-ciprofloxacin (infecton) scan. Vet. Radiol. Ultrasound 2002, 43, 178–182. [Google Scholar] [CrossRef]
- Alexander, K.; Drost, W.T.; Mattoon, J.S.; Anderson, D.E. 99mTc-ciprofloxacin in imaging of clinical infections in camelids and a goat. Vet. Radiol. Ultrasound 2005, 46, 340–347. [Google Scholar] [CrossRef]
- Wang, J.-H.; Sun, G.-F.; Zhang, J.; Shao, C.-W.; Zuo, C.-J.; Hao, J.; Zheng, J.-M.; Feng, X.-Y. Infective severe acute pancreatitis: A Comparison of 99mTc-ciprofloxacin scintigraphy and computed tomography. World J. Gastroenterol. 2013, 19, 4897–4906. [Google Scholar] [CrossRef]
- Sarda, L.; Saleh-Mghir, A.; Peker, C.; Meulemans, A.; Crémieux, A.-C.; Le Guludec, D. Evaluation of 99mTc-ciprofloxacin scintigraphy in a rabbit model of staphylococcus aureus prosthetic joint infection. J. Nucl. Med. 2002, 43, 239–245. [Google Scholar]
- Brunner, M.; Langer, O.; Dobrozemsky, G.; Müller, U.; Zeitlinger, M.; Mitterhauser, M.; Wadsak, W.; Dudczak, R.; Kletter, K.; Müller, M. [18F]Ciprofloxacin, a new positron emission tomography tracer for noninvasive assessment of the tissue distribution and pharmacokinetics of ciprofloxacin in humans. Antimicrob. Agents Chemother. 2004, 48, 3850–3857. [Google Scholar] [CrossRef] [Green Version]
- Langer, O.; Brunner, M.; Zeitlinger, M.; Ziegler, S.; Müller, U.; Dobrozemsky, G.; Lackner, E.; Joukhadar, C.; Mitterhauser, M.; Wadsak, W.; et al. In vitro and in vivo evaluation of [18F]Ciprofloxacin for the imaging of bacterial infections with PET. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Qadir, M.A.; Rasheed, R.; Ahmad, A.; Shafiq, M.I.; Ahmed, M.; Noreen, S.; Ali, A.; Shahzadi, S.K.; Javed, M. A new method for synthesis of 99mTc-enorfloxacin: An infection imaging agent. Lat. Am. J. Pharm. 2016, 35, 259–264. [Google Scholar]
- Siaens, R.H.; Rennen, H.J.; Boerman, O.C.; Dierckx, R.; Slegers, G. Synthesis and comparison of 99mTc-enrofloxacin and 99mTc-ciprofloxacin. J. Nucl. Med. 2004, 45, 2088–2094. [Google Scholar] [PubMed]
- Sazonova, S.I.; Lishmanov, Y.B.; Varlamova, N.V.; Skuridin, V.S.; Ilushenkova, Y.N.; Karpova, M.R.; Nesterov, Y.A. Synthesis and experimental study of norfloxacin labeled with technecium-99m as a potential agent for infection imaging. Iran. J. Nucl. Med. 2015, 23, 73–81. [Google Scholar]
- Ibrahim, I.T.; Motaleb, M.A.; Attalah, K.M. Synthesis and biological distribution of 99mTc-norfloxacin complex, a novel agent for detecting sites of infection. J. Radioanal. Nucl. Chem. 2010, 285, 431–436. [Google Scholar] [CrossRef]
- Fischman, A.J.; Livni, E.; Babich, J.; Alpert, N.M.; Liu, Y.Y.; Thom, E.; Cleeland, R.; Prosser, B.L.; Callahan, R.J.; Correia, J.A. Pharmacokinetics of 18F-labeled fleroxacin in rabbits with escherichia coli infections, studied with positron emission tomography. Antimicrob. Agents Chemother. 1992, 36, 2286–2292. [Google Scholar] [CrossRef] [Green Version]
- Bermingham, A.; Derrick, J.P. The folic acid biosynthesis pathway in bacteria: evaluation of potential for antibacterial drug discovery. Bioessays 2002, 24, 637–648. [Google Scholar] [CrossRef]
- Sellmyer, M.A.; Lee, I.; Hou, C.; Lieberman, B.P.; Zeng, C.; Mankoff, D.A.; Mach, R.H. Quantitative PET reporter gene imaging with [11C]trimethoprim. Molecules 2017, 25, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Rashid, U.; Ahmad, W.; Hassan, S.F.; Qureshi, N.A.; Niaz, B.; Muhammad, B.; Imdad, S.; Sajid, M. Design, synthesis, antibacterial activity and docking study of some new trimethoprim derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 5749–5753. [Google Scholar] [CrossRef]
- Mutch, C.A.; Ordonez, A.A.; Qin, H.; Parker, M.; Bambarger, L.E.; Villanueva-Meyer, J.E.; Blecha, J.; Carroll, V.; Taglang, C.; Flavell, R.; et al. [11C]Para-aminobenzoic acid: A Positron emission tomography tracer targeting bacteria-specific metabolism. ACS Infect. Dis. 2018, 4, 1067–1072. [Google Scholar] [CrossRef]
- Zhang, Z.; Ordonez, A.A.; Wang, H.; Li, Y.; Gogarty, K.R.; Weinstein, E.A.; Daryaee, F.; Merino, J.; Yoon, G.E.; Kalinda, A.S.; et al. Positron emission tomography imaging with 2-[18F]F- P-aminobenzoic acid detects staphylococcus aureus infections and monitors drug response. ACS Infect. Dis. 2018, 4, 1635–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namavari, M.; Gowrishankar, G.; Srinivasan, A.; Gambhir, S.S.; Haywood, T.; Beinat, C. A Novel synthesis of 6″-[18F]-fluoromaltotriose as a PET tracer for imaging bacterial infection. J. Label. Comp. Radiopharm. 2018, 61, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, A.A.; Bambarger, L.E.; Murthy, N.; Wilson, D.M.; Jain, S.K. Bacterial imaging. In Imaging Infections: From Bench to Bedside; Jain, S.K., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 149–172. [Google Scholar]
- Mcphee, J.B.; Hancock, R.E.W. Function and therapeutic potential of host defence peptides. J. Pept. Sci. 2005, 11, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Glukhov, E.; Stark, M.; Burrows, L.L.; Deber, C.M. Basis for Selectivity of Cationic Antimicrobial Peptides for Bacterial Versus Mammalian Membranes. J. Biol. Chem. 2005, 280, 33960–33967. [Google Scholar] [CrossRef] [Green Version]
- Welling, M.M.; Paulusma-Annema, A.; Balter, H.S.; Pauwels, E.; Nibbering, P.H. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur. J. Nucl. Med. 2000, 27, 292–301. [Google Scholar] [CrossRef]
- Pauwels, E.; Welling, M.M.; Nibbering, P.H.; Lupetti, A.; Balter, H.S. Tc-99m-labeled antimicrobial peptides for detection of bacterial and candida albicans infections—Reply. J. Nucl. Med. 2002, 43, 1126–1127. [Google Scholar]
- Gandomkar, M.; Najafi, R.; Shafiei, M.; Mazidi, M.; Goudarzi, M.; Mirfallah, S.H.; Ebrahimi, F.; Heydarpor, H.R.; Abdie, N. Clinical evaluation of antimicrobial peptide [(99m)Tc/Tricine/HYNIC(0)]Ubiquicidin 29-41 as a human-specific infection imaging agent. Nucl. Med. Biol. 2009, 36, 199–205. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bhatt, J.; Shinto, A.; Korde, A.; Kumar, M.; Kamaleshwaran, K.; Joseph, J.; Sarma, H.D.; Dash, A. 68Ga-NOTA-ubiquicidin fragment for PET imaging of infection: From bench to bedside. J. Pharm. Biomed. Anal. 2018, 159, 245–251. [Google Scholar] [CrossRef]
- Ebenhan, T.; Zeevaart, J.R.; Venter, J.D.; Govender, T.; Kruger, G.H.; Jarvis, N.V.; Sathekge, M.M. Preclinical evaluation of 68ga-labeled 1,4,7-triazacyclononane- 1,4,7-triacetic acid-ubiquicidin as a radioligand for PET infection imaging. J. Nucl. Med. 2014, 55, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Vilche, M.; Reyes, A.L.; Vasilskis, E.; Oliver, P.; Balter, H.; Engler, H. 68Ga-NOTA-UBI-29-41 as a PET Tracer for Detection of Bacterial Infection. J. Nucl. Med. 2016, 57, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Follacchio, G.A.; Pala, A.; Scaccianoce, S.; Monteleone, F.; Colletti, P.M.; Rubello, D.; Liberatore, M. In vivo microbial targeting of 99mTc-labeled human Β-defensin-3 in a rat model of infection. Clin. Nucl. Med. 2019, 44, e602–e606. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Singh, B.; Koul, A.; Mishra, A.K.; Robu, S.; Kaur, A.; Ghai, A.; Caplash, N.; Wester, H.-J. Radiosynthesis and pre-clinical evaluation of [68Ga] labeled antimicrobial peptide fragment GF-17 as a potential infection imaging PET radiotracer. Appl. Radiat. Isot. 2019, 149, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, M.; Pala, A.; Scaccianoce, S.; Anagnostou, C.; Di Tondo, U.; Calandri, E.; D’Elia, P.; Gross, M.D.; Rubello, D. Microbial targeting of 99mTc-labeled recombinant human beta-defensin-3 in an animal model of infection: A feasibility pilot study. J. Nucl. Med. 2009, 50, 823–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanaswamy, V.K.; Albericio, F.; Coovadia, Y.M.; Kruger, H.G.; Maguire, G.E.M.; Pillay, M.; Govender, T. Total synthesis of a depsidomycin analogue by convergent solid-phase peptide synthesis and macrolactonization strategy for antitubercular activity. J. Pept. Sci. 2011, 17, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Ebenhan, T.; Mokaleng, B.; Venter, J.; Kruger, H.; Zeevaart, J.; Sathekge, M. Preclinical assessment of a 68Ga-DOTA-functionalized depsipeptide as a radiodiagnostic infection imaging agent. Molecules 2017, 22, 1403. [Google Scholar] [CrossRef] [Green Version]
- Mokaleng, B.B.; Ebenhan, T.; Ramesh, S.; Govender, T.; Kruger, H.G.; Parboosing, R.; Hazari, P.P.; Mishra, A.K.; Marjanovic-Painter, B.; Zeevaart, J.R.; et al. Synthesis, 68Ga-radiolabeling, and preliminary in vivo assessment of a depsipeptide-derived compound as a potential PET/CT infection imaging agent. Biomed. Res. Int. 2015, 2015, 284354. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.P.; Gellman, S.H.; DeGrado, W.F. Β-peptides: From structure to function. Chem. Rev. 2001, 101, 3219–3232. [Google Scholar] [CrossRef]
- Seebach, D.; Beck, A.K.; Bierbaum, D.J. The world of beta- and gamma-peptides comprised of homologated proteinogenic amino acids and other components. Chem. Biodivers. 2004, 1, 1111–1239. [Google Scholar] [CrossRef]
- Zuckermann, R.N. Peptoid Origins. Biopolymers 2010, 96, 545–555. [Google Scholar] [CrossRef]
- Zuckermann, R.; Kodadek, T. Peptoids as Potential Therapeutics. Curr. Opin. Mol. 2009, 11, 299–307. [Google Scholar]
- Schafmeister, C.E.; Brown, Z.Z.; Gupta, S. Shape-Programmable Macromolecules. Acc. Chem. Res. 2008, 41, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Northrup, J.D.; Mancini, G.; Purcell, C.R.; Schafmeister, C.E. Development of spiroligomer-peptoid hybrids. J. Org. Chem. 2017, 82, 13020–13033. [Google Scholar] [CrossRef] [PubMed]
- Verdine, G.L.; Hilinski, G.J. Stapled Peptides for Intracellular Drug Targets, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 503, pp. 3–33. [Google Scholar]
- Schafmeister, C.E.; Po, J.; Verdine, G.L. An all-hydrocarbon cross-linking system for enhancing the helicity and metabolic stability of peptides. J. Am. Chem. Soc. 2000, 122, 5891–5892. [Google Scholar] [CrossRef]
- Sarnowski, M.P.; Kang, C.W.; Elbatrawi, Y.M.; Wojtas, L.; Del Valle, J.R. Peptide N-amination supports Β-sheet conformations. Angew. Chem. 2017, 129, 2115–2118. [Google Scholar] [CrossRef]
- Kang, C.W.; Sarnowski, M.P.; Elbatrawi, Y.M.; Del Valle, J.R. Access to enantiopure A-hydrazino acids for N-amino peptide synthesis. J. Org. Chem. 2017, 82, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.H.; Young, L.S.; Hansen, W.P.; Nedelman, M.; Wilkinson, R.; Nelles, M.J.; Callahan, R.; Khaw, B.A.; Strauss, H.W. Specific and nonspecific imaging of localized fisher immunotype 1 pseudomonas aeruginosa infection with radiolabeled monoclonal antibody. J. Nucl. Med. 1988, 29, 651–656. [Google Scholar] [PubMed]
- Hotze, A.L.; Briele, B.; Overbeck, B.; Kropp, J.; Gruenwald, F.; Mekkawy, M.A.; Von Smekal, A.; Moeller, F.; Biersack, H.J. Tc-99m-Labeled Antigranulocyte Antibodies in Suspected Bone-Infections. J. Nucl. Med. 1992, 33, 526–531. [Google Scholar]
- Bitkover, C.Y.; Gardlund, B.; Larsson, S.A.; Aberg, B.; Jacobsson, H. Diagnosing sternal wound infections with Tc-99m-labeled monoclonal granulocyte antibody scintigraphy. Ann. Thorac. Surg. 1996, 62, 1412–1416. [Google Scholar] [CrossRef]
- Pastrana, F.R.; Thompson, J.M.; Heuker, M.; Hoekstra, H.; Dillen, C.A.; Ortines, R.V.; Ashbaugh, A.G.; Pickett, J.E.; Linssen, M.D.; Bernthal, N.M.; et al. Noninvasive optical and nuclear imaging of staphylococcus-specific infection with a human monoclonal antibody-based probe. Virulence 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Pickett, J.E.; Thompson, J.M.; Sadowska, A.; Tkaczyk, C.; Sellman, B.R.; Minola, A.; Corti, D.; Lanzavecchia, A.; Miller, L.S.; Thorek, D.L. Molecularly specific detection of bacterial lipoteichoic acid for diagnosis of prosthetic joint infection of the bone. Bone Res. 2018, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Welling, M.; Feitsma, H.I.J.; Calame, W.; Ensing, G.J.; Goedemans, W.; Pauwels, E.K.J. Optimized localization of bacterial infections with technetium-99m labelled human immunoglobulin after protein charge selection. Eur. J. Nucl. Med. 1994, 21, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Calame, W.; Welling, M.; Feitsma, H.I.J.; Goedemans, W.T.; Pauwels, E.K.J. Contribution of phagocytic cells and bacteria to the accumulation of technetium-99m labelled polyclonal human immunoglobulin at sites of inflammation. Eur. J. Nucl. Med. 1995, 22, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Vugts, D.J.; van Dongen, G.A.M.S. Immunoglobulins as radiopharmaceutical vectors. In Radiopharmaceutical Chemistry; Springer: Cham, Switzerland, 2019; Volume 8, pp. 163–179. [Google Scholar]
- Zettlitz, K.A.; Tavaré, R.; Tsai, W.-T.K.; Yamada, R.E.; Ha, N.S.; Collins, J.; van Dam, R.M.; Timmerman, J.M.; Wu, A.M. 18F-labeled anti-human CD20 cys-diabody for same-day immunoPET in a model of aggressive B cell lymphoma in human CD20 transgenic mice. Eur. J. Nucl. Med. 2019, 46, 489–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandit-Taskar, N.; Postow, M.; Hellmann, M.; Harding, J.; Barker, C.; O’Donoghue, J.; Ziolkowska, M.; Ruan, S.; Lyashchenko, S.; Tsai, F.; et al. First-in-human imaging with 89Zr-Df-IAB22M2C Anti-CD8 minibody in patients with solid malignancies: Preliminary pharmacokinetics, biodistribution, and lesion targeting. J. Nucl. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zettlitz, K.A.; Tsai, W.-T.K.; Knowles, S.M.; Salazar, F.B.; Kobayashi, N.; Reiter, R.E.; Wu, A.M. [89Zr]A2cDb immuno-PET of prostate cancer in a human prostate stem cell antigen knock-in (hPSCA KI) syngeneic model. Mol. Imaging Biol. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Carnazza, K.E.; Carlin, S.D.; Cho, A.; Edwards, K.J.; Sevak, K.K.; Glaser, J.M.; de Stanchina, E.; Janjigian, Y.Y.; Lewis, J.S. 89Zr-DFO-AMG102 immuno-PET to determine local hepatocyte growth factor protein levels in tumors for enhanced patient selection. J. Nucl. Med. 2017, 58, 1386–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freise, A.C.; Zettlitz, K.A.; Salazar, F.B.; Lu, X.; Tavaré, R.; Wu, A.M. ImmunoPET imaging of murine CD4 + T cells using anti-CD4 Cys-diabody: Effects of protein dose on T cell function and imaging. Mol. Imaging Biol. 2017, 19, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Tavaré, R.; McCracken, M.N.; Zettlitz, K.A.; Knowles, S.M.; Salazar, F.B.; Olafsen, T.; Witte, O.N.; Wu, A.M. Engineered antibody fragments for immuno-PET imaging of endogenous CD8 + T cells in VIVO. Proc. Natl. Acad. Sci. USA 2014, 111, 1108–1113. [Google Scholar] [CrossRef] [Green Version]
- Tavaré, R.; Escuin-Ordinas, H.; Mok, S.; McCracken, M.N.; Zettlitz, K.A.; Salazar, F.B.; Witte, O.N.; Ribas, A.; Wu, A.M. An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res. 2016, 76, 73–82. [Google Scholar] [CrossRef]







| Isotope | T1/2 | Attachment | Production | Decay Type | Decay Energy | Ideal Setting |
|---|---|---|---|---|---|---|
| 11C | 20.4 min | Covalent | Cyclotron | β+ | 1.0 MeV | Research/Clinical Imaging |
| 18F | 110 min | Covalent | Cyclotron | β+ | 0.6 MeV | Clinical Imaging |
| 68Ga | 67.6 min | Chelation | Generator | β+, γ | 1.9 MeV, 1.1 MeV | Clinical Imaging * |
| 76Br | 16.2 h | Covalent | Cyclotron | β+ | 0.8–3.9 MeV | Clinical Imaging |
| 89Zr | 78.4 h | Chelation | Cyclotron | β+ | 0.9 MeV | Clinical Imaging |
| 90Y | 64.1 h | Chelation | Separation | β−, γ | 2.3 MeV, 2.2 MeV | Therapy |
| 99mTc | 6.0 h | Chelation | Generator | γ | 141 keV | Clinical Imaging * |
| 111In | 2.8 d | Chelation | Cyclotron | γ, EC | 245 keV | Clinical Imaging |
| 123I | 13.2 h | Covalent | Cyclotron | γ, EC | 159 keV | Clinical Imaging |
| 124I | 4.2 d | Covalent | Cyclotron | β+ | 1.5–2.1 MeV | Clinical Imaging |
| 125I | 59.4 d | Covalent | Cyclotron | γ, EC | 35 keV | Preclinical Imaging |
| 131I | 8.0 d | Covalent | Cyclotron | β−, γ | 0.6 MeV, 364 keV | Imaging/Therapy |
| 177Lu | 6.7 d | Chelation | Cyclotron | β−, γ | 0.5 MeV, 208 keV | Therapy |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Northrup, J.D.; Mach, R.H.; Sellmyer, M.A. Radiochemical Approaches to Imaging Bacterial Infections: Intracellular versus Extracellular Targets. Int. J. Mol. Sci. 2019, 20, 5808. https://doi.org/10.3390/ijms20225808
Northrup JD, Mach RH, Sellmyer MA. Radiochemical Approaches to Imaging Bacterial Infections: Intracellular versus Extracellular Targets. International Journal of Molecular Sciences. 2019; 20(22):5808. https://doi.org/10.3390/ijms20225808
Chicago/Turabian StyleNorthrup, Justin D., Robert H. Mach, and Mark A. Sellmyer. 2019. "Radiochemical Approaches to Imaging Bacterial Infections: Intracellular versus Extracellular Targets" International Journal of Molecular Sciences 20, no. 22: 5808. https://doi.org/10.3390/ijms20225808
APA StyleNorthrup, J. D., Mach, R. H., & Sellmyer, M. A. (2019). Radiochemical Approaches to Imaging Bacterial Infections: Intracellular versus Extracellular Targets. International Journal of Molecular Sciences, 20(22), 5808. https://doi.org/10.3390/ijms20225808