Stability and Fermentability of Green Tea Flavonols in In-Vitro-Simulated Gastrointestinal Digestion and Human Fecal Fermentation
Abstract
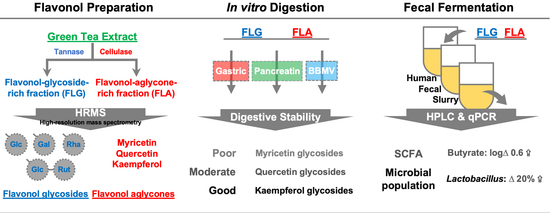
:1. Introduction
2. Results and Discussion
2.1. Stabilities of Kaempferol, Myricetin, and Quercetin in In Vitro Digestion
2.2. Gastrointestinal Stabilities of FLG and FLA in In Vitro Digestion
2.3. Modulating Interaction of FLG and FLA on Microbial Populations in In Vitro Fecal Fermentation
2.4. Effects of FLG and FLA on SCFAs and Lactate Production in In Vitro Fecal Fermentation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of FLG and FLA
3.3. Preparation of BBMVs
3.4. In Vitro Digestibility of Pure Flavonols, FLG, and FLA
3.5. In Vitro Fecal Batch Fermentation of FLG and FLA
3.6. Enumeration of Intestinal Bacteria Using qRT-PCR
3.7. Determination of Flavonoids, Lactate, and SCFAs Using HPLC Systems
3.8. Statistical Analysis
3.9. Ethical Statement
3.10. Data Availability
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BBMV | brush border membrane vesicle |
| DMSO | dimethyl sulfoxide |
| FLA | flavonol-aglycone-rich fraction |
| FLG | flavonol-glycoside-rich fraction |
| HPLC | high-performance liquid chromatography |
| LPH | lactase-phlorizin hydrolase |
| PBS | phosphate-buffered saline |
| qPCR | quantitative polymerase chain reaction |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| SCFA | short-chain fatty acid |
| SGF | simulated gastric fluid |
| SIF | simulated intestinal fluid |
References
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, H.; Héritier, J.; Andlauer, W. Determination of catechins and flavonol glycosides in Chinese tea varieties. Food Chem. 2012, 132, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Engelhardt, U.H.; Thräne, C.; Maiwald, B.; Stark, J. Determination of flavonol glycosides in green tea, oolong tea and black tea by UHPLC compared to HPLC. Food Chem. 2015, 183, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Monobe, M.; Nomura, S.; Ema, K.; Matsunaga, A.; Nesumi, A.; Yoshida, K.; Maeda-Yamamoto, M.; Horie, H. Quercetin glycosides-rich tea cultivars (Camellia sinensis L.) in Japan. Food Sci. Technol. Res. 2015, 21, 333–340. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, C.Y. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit. Rev. Food Sci. Nutr. 2004, 44, 253–273. [Google Scholar] [CrossRef]
- Lea, M.A. Flavonol regulation in tumor cells. J. Cell. Biochem. 2015, 116, 1190–1194. [Google Scholar] [CrossRef]
- Nomura, S.; Monobe, M.; Ema, K.; Matsunaga, A.; Maeda-Yamamoto, M.; Horie, H. Effects of flavonol-rich green tea cultivar (Camellia sinensis L.) on plasma oxidized LDL levels in hypercholesterolemic mice. Biosci. Biotechnol. Biochem. 2016, 80, 360–362. [Google Scholar] [CrossRef]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrias, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.R.; Dumont, J.; et al. Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef]
- Sanchez-Bridge, B.; Lévèques, A.; Li, H.; Bertschy, E.; Patin, A.; Actis-Goretta, L. Modulation of (−)-epicatechin metabolism by coadministration with other polyphenols in Caco-2 cell model. Drug Metab. Dispos. 2015, 43, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Landis-Piwowar, K.; Chen, D.; Chan, T.H.; Dou, Q.P. Inhibition of catechol-O-methyltransferase activity in human breast cancer cells enhances the biological effect of the green tea polyphenol (−)-EGCG. Oncol. Rep. 2010, 24, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.-O.; Lee, S.-B.; Jeong, K.-H.; Song, J.-H.; Kim, S.; Joo, K.-M.; Jeong, H.W.; Choi, J.K.; Kim, J.K.; Kim, W.G.; et al. Quercetin and fisetin enhanced small intestine cellular uptake and plasma levels of epi-catechins in vitro and in vivo model. Food Funct. 2018, 9, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; van Trijp, J.M.P.; Buysman, M.N.C.P.; van der Gaag, M.S.; Mengelers, M.J.B.; de Vries, J.H.M.; Katan, M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997, 418, 152–156. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Bijsman, M.N.C.P.; van Gameren, Y.; Cnossen, E.P.J.; de Vries, J.H.M.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radical Res. 1999, 31, 569–573. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Vree, T.B.; Katan, M.B. Bioavailabilities of quercetin-3-glucoside and quercetin-4’-glucoside do not differ in humans. J. Nutr. 2000, 130, 1200–1203. [Google Scholar] [CrossRef]
- Walle, T.; Otake, Y.; Walle, U.K.; Wilson, F.A. Quercetin glucosides are completely hydrolyzed in ileostomy patients before absorption. J. Nutr. 2000, 130, 2658–2661. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Edwards, C.A.; Crozier, A. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic. Res. 2006, 40, 1035–1046. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Simons, A.L.; Renouf, M.; Hendrich, S.; Murphy, P.A. Human gut microbial degradation of flavonoids: Structure−function relationships. J. Agric. Food. Chem. 2005, 53, 4258–4263. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food. Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Wang, C.-g.; Wang, W.-q.; Shi, C.-y.; Xiong, W.; Wang, M.-d.; Fang, J.-g. Gastrointestinal stability of dihydromyricetin, myricetin, and myricitrin: An in vitro investigation. Int. J. Food Sci. Nutr. 2017, 68, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Lin, G.; Xie, Y.; Ma, P.; Li, G.; Meng, Q.; Wu, T. Preformulation studies of myricetin: A natural antioxidant flavonoid. Pharmazie 2014, 69, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.-H. Degradation kinetics of fisetin and quercetin in solutions affected by medium pH, temperature and co-existed proteins. J. Serb. Chem. Soc. 2016, 81, 243–253. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Kadouh, H.; Zhou, K. Phenolic compounds and antioxidant properties of gooseberry as affected by in vitro digestion. LWT-Food Sci. Technol. 2013, 51, 417–422. [Google Scholar] [CrossRef]
- Rha, C.-S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.-O. Antioxidative, anti-inflammatory, and anticancer effects of purified flavonol glycosides and aglycones in green tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the flavonoid C-glycosides and health benefits. Crit. Rev. Food Sci. Nutr. 2016, 56(Sup. 1), S29–S45. [Google Scholar] [CrossRef]
- Goh, L.M.L.; Barlow, P.J. Flavonoid recovery and stability from Ginkgo biloba subjected to a simulated digestion process. Food Chem. 2004, 86, 195–202. [Google Scholar] [CrossRef]
- Németh, K.; Plumb, G.W.; Berrin, J.-G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Boyer, J.; Brown, D.; Liu, R.H. In vitro digestion and lactase treatment influence uptake of quercetin and quercetin glucoside by the Caco-2 cell monolayer. Nutr. J. 2005, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, E.J.; Watson, D.G. In vitro glucuronidation of kaempferol and quercetin by human UGT 1A9 microsomes. FEBS Lett. 2000, 471, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Sugiyama, Y.; Sakano, T.; Ohigashi, H. Flavonols enhanced production of anti-inflammatory substance(s) by Bifidobacterium adolescentis: Prebiotic actions of galangin, quercetin, and fisetin. BioFactors 2013, 39, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, A.W.; Duncan, S.H.; Leitch, E.C.M.; Child, M.W.; Flint, H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [Green Version]
- El Aidy, S.; Derrien, M.; Merrifield, C.A.; Levenez, F.; Doré, J.; Boekschoten, M.V.; Dekker, J.; Holmes, E.; Zoetendal, E.G.; van Baarlen, P.; et al. Gut bacteria-host metabolic interplay during conventionalisation of the mouse germfree colon. ISME J. 2013, 7, 743–755. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Siebold, M.; Frieling, P.v.; Joppien, R.; Rindfleisch, D.; Schügerl, K.; Röper, H. Comparison of the production of lactic acid by three different Lactobacilli and its recovery by extraction and electrodialysis. Process Biochem. 1995, 30, 81–95. [Google Scholar] [CrossRef]
- Adamberg, S.; Tomson, K.; Vija, H.; Puurand, M.; Kabanova, N.; Visnapuu, T.; Jõgi, E.; Alamäe, T.; Adamberg, K. Degradation of fructans and production of propionic acid by Bacteroides thetaiotaomicron are enhanced by the shortage of amino acids. Front. Nutr. 2014, 1, 21. [Google Scholar] [CrossRef] [Green Version]
- Bränning, C.E.; Nyman, M.E. Malt in combination with Lactobacillus rhamnosus increases concentrations of butyric acid in the distal colon and serum in rats compared with other barley products but decreases viable counts of cecal bifidobacteria. J. Nutr. 2011, 141, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [Green Version]
- Aura, A.-M.; O’Leary, K.A.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimiä, R.; Nuutila, A.M.; Oksman-Caldentey, K.-M.; Poutanen, K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J. Agric. Food. Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef]
- Hein, E.-M.; Rose, K.; van’t Slot, G.; Friedrich, A.W.; Humpf, H.-U. Deconjugation and degradation of flavonol glycosides by pig cecal microbiota characterized by fluorescence in situ hybridization (FISH). J. Agric. Food. Chem. 2008, 56, 2281–2290. [Google Scholar] [CrossRef]
- Choi, E.-H.; Rha, C.-S.; Balusamy, S.R.; Kim, D.-O.; Shim, S.-M. Impact of bioconversion of gallated catechins and flavonol glycosides on bioaccessibility and intestinal cellular uptake of catechins. J. Agric. Food. Chem. 2019, 67, 2331–2339. [Google Scholar] [CrossRef]
- Gnoth, M.J.; Kunz, C.; Kinne-Saffran, E.; Rudloff, S. Human milk oligosaccharides are minimally digested in vitro. J. Nutr. 2000, 130, 3014–3020. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Mandalari, G.; Nueno-Palop, C.; Bisignano, G.; Wickham, M.S.J.; Narbad, A. Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270. [Google Scholar] [CrossRef] [Green Version]
- Seong, H.; Bae, J.-H.; Seo, J.S.; Kim, S.-A.; Kim, T.-J.; Han, N.S. Comparative analysis of prebiotic effects of seaweed polysaccharides laminaran, porphyran, and ulvan using in vitro human fecal fermentation. J. Funct. Foods 2019, 57, 408–416. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Compound | Digestive Conditions | ||||
|---|---|---|---|---|---|
| Control | Gastric | Pancreatin | BBMV a | Pancreatin + BBMV b | |
| Kaempferol | 99.20 ± 4.30 Ac | 93.40 ± 2.60 A | 65.50 ± 0.50 BC | 72.23 ± 0.63 B | 54.97 ± 0.58 C |
| Myricetin | 101.60 ± 1.53 A | 98.97 ± 2.36 A | n.d. d | n.d. | n.d. |
| Quercetin | 99.07 ± 5.48 A | 96.47 ± 1.35 A | 9.23 ± 0.35 B | 4.47 ± 0.12 C | n.d. |
| Peak No. a | Compound b | Peak No. | Compound |
|---|---|---|---|
| 1 | Apigenin-6-C-glucosyl-8-C-arabinoside | 9 | Quercetin-3-O-galactoside |
| 2 | Myricetin-3-O-galactoside | 10 | Quercetin-3-O-glucoside |
| 3 | Myricetin-3-O-glucoside | 11 | Kaempferol-3-O-glucosylrutinoside |
| 4 | Quercetin-3-O-galactosylrutinoside | 12 | Kaempferol-3-O-rhamnosylgalactoside |
| 5 | Quercetin-3-O-glucosylrutinoside | 13 | Kaempferol-3-O-rhamnosylglucoside |
| 6 | Quercetin-3-O-rhamnosylgalactoside | 14 | Myricetin |
| 7 | Quercetin-3-O-rhamnosylglucoside | 15 | Quercetin |
| 8 | Apigenin-6-C-glucoside or its isomer | 16 | Kaempferol |
| Conditions a | Digestive Enzyme | Buffer | Stopping Solution | Reaction Temperature and Time |
|---|---|---|---|---|
| Gastric | pepsin (160 μL) | SGF b (750 μL), 0.3 M CaCl2 (0.5 μL), 1 M HCl (1.0 μL), water (88.5 μL); pH 3.0 | solvent 1 c (400 μL) + water (100 μL) | 37 °C, 2 h |
| Pancreatin | pancreatin (250 μL) | SIF d (550 μL), bile (125 μL), 0.3 M CaCl2 (2 μL), 1 M HCl (10 μL), water (63 μL); pH 7.0 | solvent 2 e (400 μL) + water (100 μL) | 37 °C, 2 h |
| BBMVf | BBMV (100 μL) | SIF (550 μL), bile (125 μL), 0.3 M CaCl2 (2 μL),1 M HCl (10 μL), water (213 μL); pH 7.0 | solvent 2 (400 μL) + water (100 μL) | 37 °C, 4 h |
| Pancreatin + BBMV | pancreatin (250 μL), BBMV (100 μL) | SIF (550 μL), bile (125 μL), 0.3 M CaCl2 (2 μL),1 M HCl (10 μL), water (63 μL); pH 7.0 | solvent 2 (400 μL) | 37 °C, 2 h or 37 °C, 4 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rha, C.-S.; Seong, H.; Jung, Y.S.; Jang, D.; Kwak, J.-G.; Kim, D.-O.; Han, N.S. Stability and Fermentability of Green Tea Flavonols in In-Vitro-Simulated Gastrointestinal Digestion and Human Fecal Fermentation. Int. J. Mol. Sci. 2019, 20, 5890. https://doi.org/10.3390/ijms20235890
Rha C-S, Seong H, Jung YS, Jang D, Kwak J-G, Kim D-O, Han NS. Stability and Fermentability of Green Tea Flavonols in In-Vitro-Simulated Gastrointestinal Digestion and Human Fecal Fermentation. International Journal of Molecular Sciences. 2019; 20(23):5890. https://doi.org/10.3390/ijms20235890
Chicago/Turabian StyleRha, Chan-Su, Hyunbin Seong, Young Sung Jung, Davin Jang, Jun-Gu Kwak, Dae-Ok Kim, and Nam Soo Han. 2019. "Stability and Fermentability of Green Tea Flavonols in In-Vitro-Simulated Gastrointestinal Digestion and Human Fecal Fermentation" International Journal of Molecular Sciences 20, no. 23: 5890. https://doi.org/10.3390/ijms20235890
APA StyleRha, C. -S., Seong, H., Jung, Y. S., Jang, D., Kwak, J. -G., Kim, D. -O., & Han, N. S. (2019). Stability and Fermentability of Green Tea Flavonols in In-Vitro-Simulated Gastrointestinal Digestion and Human Fecal Fermentation. International Journal of Molecular Sciences, 20(23), 5890. https://doi.org/10.3390/ijms20235890