Honey-Mediated Wound Healing: H2O2 Entry through AQP3 Determines Extracellular Ca2+ Influx
Abstract
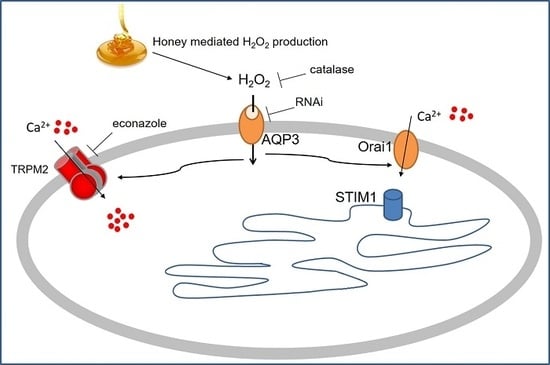
:1. Introduction
2. Results
2.1. Honey Induces Ca2+ Signals in a Dose-Dependent Manner
2.2. The Ca2+ Response to Manuka Honey Requires Extracellular Ca2+ Entry and the Intracellular Production of Hydrogen Peroxide
2.3. Role of Specific Inhibitors of Ca2+ Entry
2.4. Role of AQP3 in H2O2 Influx
3. Discussion
4. Materials and Methods
4.1. Honey Samples
4.2. Cell Culture and Reagents
4.3. Measurements of Free Cytosolic Ca2+ Concentration ([Ca2+]i)
4.4. H2O2 Assays
4.5. Measurement of Intracellular ROS
4.6. Quantitative Reverse Transcriptase PCR (qRT-PCR) and RNA Interference (siRNA)
4.7. Immunoblotting
4.8. Scratch Wound Test
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Zumla, A.; Lulat, A. Honey—A remedy rediscovered. J. R. Soc. Med. 1989, 82, 384–385. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Ranzato, E.; Burlando, B. Signaling pathways in wound repair. In Wound Healing: Process, Phases and Promoting; Middleton, J.E., Ed.; Nova Publishers Inc.: New York, NY, USA, 2011; Volume 7, pp. 123–135. [Google Scholar]
- Ranzato, E.; Martinotti, S.; Burlando, B. Epithelial mesenchymal transition traits in honey-driven keratinocyte wound healing: Comparison among different honeys. Wound Repair Regen. 2012, 20, 778–785. [Google Scholar] [CrossRef]
- Ranzato, E.; Martinotti, S.; Burlando, B. Honey exposure stimulates wound repair of human dermal fibroblasts. Burn. Trauma 2013, 1, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Honey, Wound Repair and Regenerative Medicine. J. Funct. Biomater. 2018, 9, 34. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Walczysko, P.; Zhao, M. Intracellular Ca2+ stores are essential for injury induced Ca2+ signaling and re-endothelialization. J. Cell Physiol. 2008, 214, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Ranzato, E.; Mazzucco, L.; Patrone, M.; Burlando, B. Platelet lysate promotes in vitro wound scratch closure of human dermal fibroblasts: Different roles of cell calcium, P38, ERK and PI3K/AKT. J. Cell. Mol. Med. 2009, 13, 2030–2038. [Google Scholar] [CrossRef]
- Moccia, F.; Tanzi, F.; Munaron, L. Endothelial remodelling and intracellular calcium machinery. Curr. Mol. Med. 2014, 14, 457–480. [Google Scholar]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 1995, 80, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Hu, Q.; Corda, S.; Zweier, J.L.; Capogrossi, M.C.; Ziegelstein, R.C. Hydrogen peroxide induces intracellular calcium oscillations in human aortic endothelial cells. Circulation 1998, 97, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Bang, L.M.; Buntting, C.; Molan, P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J. Altern. Complement. Med. 2003, 9, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hecquet, C.M.; Ahmmed, G.U.; Vogel, S.M.; Malik, A.B. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ. Res. 2008, 102, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Faouzi, M.; Penner, R. Trpm2. Handb. Exp. Pharm. 2014, 222, 403–426. [Google Scholar]
- Winterbourn, C.C. Biological Production, Detection and Fate of Hydrogen Peroxide. Antioxid. Redox Signal 2018, 29, 541–551. [Google Scholar] [CrossRef]
- Kong, H.; Fan, Y.; Xie, J.; Ding, J.; Sha, L.; Shi, X.; Sun, X.; Hu, G. AQP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J. Cell Sci. 2008, 121, 4029–4036. [Google Scholar] [CrossRef] [Green Version]
- Grupe, M.; Myers, G.; Penner, R.; Fleig, A. Activation of store-operated I(CRAC) by hydrogen peroxide. Cell Calcium 2010, 48, 1–9. [Google Scholar] [CrossRef]
- Santiago, E.; Climent, B.; Munoz, M.; Garcia-Sacristan, A.; Rivera, L.; Prieto, D. Hydrogen peroxide activates store-operated Ca(2+) entry in coronary arteries. Br. J. Pharm. 2015, 172, 5318–5332. [Google Scholar]
- Oda, S.; Uchida, K.; Wang, X.Y.; Lee, J.; Shimada, Y.; Tominaga, M.; Kadowaki, M. TRPM2 contributes to antigen-stimulated Ca2+ influx in mucosal mast cells. Pflug. Arch. Eur. J. Phys. 2013, 465, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Fresia, C.; Di Buduo, C.A.; Soprano, P.M.; Moccia, F.; Balduini, C.; Zocchi, E.; De Flora, A.; Balduini, A. The Plant Hormone Abscisic Acid Is a Prosurvival Factor in Human and Murine Megakaryocytes. J. Biol. Chem. 2017, 292, 3239–3251. [Google Scholar] [CrossRef] [Green Version]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara-Chikuma, M.; Verkman, A.S. Roles of aquaporin-3 in the epidermis. J. Investig. Dermatol. 2008, 128, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Boury-Jamot, M.; Daraspe, J.; Bonte, F.; Perrier, E.; Schnebert, S.; Dumas, M.; Verbavatz, J.M. Skin aquaporins: Function in hydration, wound healing, and skin epidermis homeostasis. Handb. Exp. Pharm. 2009, 190, 205–217. [Google Scholar]
- Benga, G. On the definition, nomenclature and classification of water channel proteins (aquaporins and relatives). Mol. Asp. Med. 2012, 33, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laforenza, U.; Pellavio, G.; Marchetti, A.L.; Omes, C.; Todaro, F.; Gastaldi, G. Aquaporin-Mediated Water and Hydrogen Peroxide Transport Is Involved in Normal Human Spermatozoa Functioning. Int. J. Mol. Sci. 2016, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajah, J.R.; Chang, J.; Goettel, J.A.; Verkman, A.S.; Lencer, W.I. Aquaporin-3 mediates hydrogen peroxide-dependent responses to environmental stress in colonic epithelia. Proc. Natl. Acad. Sci. USA 2017, 114, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranzato, E.; Martinotti, S.; Balbo, V.; Boccafoschi, F.; Patrone, M.; Mazzucco, L.; Burlando, B. Platelet Lysate-Driven in Vitro Wound Healing. Wound Repair Regen. 2009, 17, A78. [Google Scholar]
- Ranzato, E.; Patrone, M.; Mazzucco, L.; Burlando, B. Platelet lysate stimulates wound repair of HaCaT keratinocytes. Br. J. Derm. 2008, 159, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Berra-Romani, R.; Raqeeb, A.; Avelino-Cruz, J.E.; Moccia, F.; Oldani, A.; Speroni, F.; Taglietti, V.; Tanzi, F. Ca2+ signaling in injured in situ endothelium of rat aorta. Cell Calcium 2008, 44, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Sammak, P.J.; Hinman, L.E.; Tran, P.O.T.; Sjaastad, M.D.; Machen, T.E. How do injured cells communicate with the surviving cell monolayer? J. Cell Sci. 1997, 110, 465–475. [Google Scholar] [PubMed]
- Lansdown, A.B.; Sampson, B.; Rowe, A. Sequential changes in trace metal, metallothionein and calmodulin concentrations in healing skin wounds. J. Anat. 1999, 195, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Martinotti, S.; Ranzato, E. Honey’s healing history. In Cellular and Molecular Mechanisms of Honey Wound Healing; Martinotti, S., Ranzato, E., Eds.; Nova Publishers Inc.: New York, NY, USA, 2014; ISBN 978-1-63117-251-9. [Google Scholar]
- Olaitan, P.B.; Adeleke, O.E.; Ola, I.O. Honey: A reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007, 7, 159–165. [Google Scholar] [PubMed]
- Nunes, P.; Demaurex, N. Redox regulation of store-operated Ca2+ entry. Antioxid. Redox Signal 2014, 21, 915–932. [Google Scholar] [CrossRef]
- Bucekova, M.; Juricova, V.; Monton, E.; Martinotti, S.; Ranzato, E.; Majtan, J. Microwave processing of honey negatively affects honey antibacterial activity by inactivation of bee-derived glucose oxidase and defensin-1. Food Chem. 2018, 240, 1131–1136. [Google Scholar] [CrossRef]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef]
- Malinouski, M.; Zhou, Y.; Belousov, V.V.; Hatfield, D.L.; Gladyshev, V.N. Hydrogen peroxide probes directed to different cellular compartments. PLoS ONE 2011, 6, e14564. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehage, E.; Eisfeld, J.; Heiner, I.; Jungling, E.; Zitt, C.; Luckhoff, A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J. Biol. Chem. 2002, 277, 23150–23156. [Google Scholar] [CrossRef] [PubMed]
- Kolisek, M.; Beck, A.; Fleig, A.; Penner, R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol. Cell 2005, 18, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Perraud, A.L.; Takanishi, C.L.; Shen, B.; Kang, S.; Smith, M.K.; Schmitz, C.; Knowles, H.M.; Ferraris, D.; Li, W.; Zhang, J.; et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J. Biol. Chem. 2005, 280, 6138–6148. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.J.; Irrinki, K.M.; Mallilankaraman, K.; Lien, Y.C.; Wang, Y.; Bhanumathy, C.D.; Subbiah, R.; Ritchie, M.F.; Soboloff, J.; Baba, Y.; et al. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J. Cell Biol. 2010, 190, 391–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinotti, S.; Calabrese, G.; Ranzato, E. Honeydew honey: Biological effects on skin cells. Mol. Cell. Biochem. 2017, 435, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, V.; Ranzato, E.; Balbo, V.; Mazzucco, L.; Cavaletto, M.; Patrone, M. Long-term effect of platelet lysate on primary fibroblasts highlighted with a proteomic approach. J. Tissue Eng. Regen. Med. 2009, 3, 531–538. [Google Scholar] [CrossRef]
- Zuccolo, E.; Lim, D.; Kheder, D.A.; Perna, A.; Catarsi, P.; Botta, L.; Rosti, V.; Riboni, L.; Sancini, G.; Tanzi, F.; et al. Acetylcholine induces intracellular Ca(2+) oscillations and nitric oxide release in mouse brain endothelial cells. Cell Calcium 2017, 66, 33–47. [Google Scholar] [CrossRef]
- Ranzato, E.; Boccafoschi, F.; Mazzucco, L.; Patrone, M.; Burlando, B. Role of ERK1/2 in platelet lysate-driven endothelial cell repair. J. Cell. Biochem. 2010, 110, 783–793. [Google Scholar] [CrossRef]
- Andrikopoulos, P.; Baba, A.; Matsuda, T.; Djamgoz, M.B.A.; Yaqoob, M.M.; Eccles, S.A. Ca2+ Influx through Reverse Mode Na+/Ca2+ Exchange Is Critical for Vascular Endothelial Growth Factor-mediated Extracellular Signal-regulated Kinase (ERK) 1/2 Activation and Angiogenic Functions of Human Endothelial Cells. J. Biol. Chem. 2011, 286, 37919–37931. [Google Scholar] [CrossRef]
- Umemura, M.; Baljinnyam, E.; Feske, S.; De Lorenzo, M.S.; Xie, L.H.; Feng, X.; Oda, K.; Makino, A.; Fujita, T.; Yokoyama, U.; et al. Store-operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS ONE 2014, 9, e89292. [Google Scholar] [CrossRef]
- Gelderblom, M.; Melzer, N.; Schattling, B.; Gob, E.; Hicking, G.; Arunachalam, P.; Bittner, S.; Ufer, F.; Herrmann, A.M.; Bernreuther, C.; et al. Transient receptor potential melastatin subfamily member 2 cation channel regulates detrimental immune cell invasion in ischemic stroke. Stroke 2014, 45, 3395–3402. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Nepal, S.; Tsukasaki, Y.; Hecquet, C.M.; Soni, D.; Rehman, J.; Tiruppathi, C.; Malik, A.B. Neutrophil Activation of Endothelial Cell-Expressed TRPM2 Mediates Transendothelial Neutrophil Migration and Vascular Injury. Circ. Res. 2017, 121, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Hecquet, C.M.; Malik, A.B. Role of H2O2-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb. Haemost. 2009, 101, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Ranzato, E.; Magnelli, V.; Martinotti, S.; Waheed, Z.; Cain, S.M.; Snutch, T.P.; Marchetti, C.; Burlando, B. Epigallocatechin-3-gallate elicits Ca2+ spike in MCF-7 breast cancer cells: Essential role of Cav3.2 channels. Cell Calcium 2014, 56, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Ranzato, E.; Martinotti, S.; Magnelli, V.; Murer, B.; Biffo, S.; Mutti, L.; Burlando, B. Epigallocatechin-3-gallate induces mesothelioma cell death via H2O2-dependent T-type Ca2+ channel opening. J. Cell. Mol. Med. 2012, 16, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [PubMed]
- Ranzato, E.; Biffo, S.; Burlando, B. Selective ascorbate toxicity in malignant mesothelioma: A redox Trojan mechanism. Am. J. Respir. Cell Mol. Biol. 2011, 44, 108–117. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]









| Target Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| β-actin | 5′-TCCCTGGAGAAGAGCTACGA-3′ | 5′-AGCACTGTGTTGGCGTACAG-3′ |
| GADPH | 5′-AATCCCATCACCATCTTCCA-3′ | 5′-TGGACTCCACGACGTACTCA-3′ |
| AQP1 | 5′-TAAGGAGAGGAAAGTTCCAG-3′ | 5′-AAAGGCAGACATACACATAC-3′ |
| AQP3 | 5′-CTGTGTATGTGTATGTCTGC-3′ | 5′-TTATGACCTGACTTCACTCC-3′ |
| AQP4 | 5′-GCTGTGATTCCAAACGGACTGATC-3′ | 5′-CTGACTCCTGTTGTCCTCCACCTC-3′ |
| AQP5 | 5′-GCTGGCACTCTGCATCTTCGC-3′ | 5′-AGGTAGAAGTAAAGGATGGCAGC-3′ |
| AQP6 | 5′-ATTGGGATCCACTTCACTG-3′ | 5′-AGTGGACTGTGAACTTCC-3′ |
| AQP8 | 5′-GGAGATAAGAGTATCTTGCAC-3′ | 5′-CTTGTCATTGCCAAATTCAG-3′ |
| AQP9 | 5′-GTATTGGTAGAAACAGGAGTC-3′ | 5′-GGACAATCAAGATGAACGTG-3′ |
| ORAI1 | 5′-ATAAGCATTTCCTGTTCTTCC-3′ | 5′-ACACATGTACACACTCAATG-3′ |
| ORAI2 | 5′-ATTCGTATAAATGACCTGCC-3′ | 5′-GTGGTGGTTAGAGGTGAC-3′ |
| ORAI3 | 5′-CTATCTTTGGAGGTTCAAGC-3′ | 5′-AGAACAAGTTTGGTGCATAG-3′ |
| STIM1 | 5′-AGTGAGGATGAGAAACTCAG-3′ | 5′-GAACTCATCACTTTCTTCCAC-3′ |
| STIM2 | 5′-GCTATTGCTAAAGATGAGGC-3′ | 5′-TCCAGAATTTTGTGGTCTAC-3′ |
| TRPM2 | 5′-CTACCTGAAGGAGAACTACC-3′ | 5′-CAACCTTATTGCTGATGTCC-3′ |
| Target Protein | Forward Sequence | Reverse Sequence |
|---|---|---|
| AQP3 | 5′-GAGCAGAUCUGAGUGGGCA-3′ | 5′-UGCCCACUCAGAUCUGCUC-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinotti, S.; Laforenza, U.; Patrone, M.; Moccia, F.; Ranzato, E. Honey-Mediated Wound Healing: H2O2 Entry through AQP3 Determines Extracellular Ca2+ Influx. Int. J. Mol. Sci. 2019, 20, 764. https://doi.org/10.3390/ijms20030764
Martinotti S, Laforenza U, Patrone M, Moccia F, Ranzato E. Honey-Mediated Wound Healing: H2O2 Entry through AQP3 Determines Extracellular Ca2+ Influx. International Journal of Molecular Sciences. 2019; 20(3):764. https://doi.org/10.3390/ijms20030764
Chicago/Turabian StyleMartinotti, Simona, Umberto Laforenza, Mauro Patrone, Francesco Moccia, and Elia Ranzato. 2019. "Honey-Mediated Wound Healing: H2O2 Entry through AQP3 Determines Extracellular Ca2+ Influx" International Journal of Molecular Sciences 20, no. 3: 764. https://doi.org/10.3390/ijms20030764
APA StyleMartinotti, S., Laforenza, U., Patrone, M., Moccia, F., & Ranzato, E. (2019). Honey-Mediated Wound Healing: H2O2 Entry through AQP3 Determines Extracellular Ca2+ Influx. International Journal of Molecular Sciences, 20(3), 764. https://doi.org/10.3390/ijms20030764