Change in Renal Glomerular Collagens and Glomerular Filtration Barrier-Related Proteins in a Dextran Sulfate Sodium-Induced Colitis Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Progression of DSS-Colitis
2.2. Renal Morphology and Histological Changes in DSS-Colitis Mice
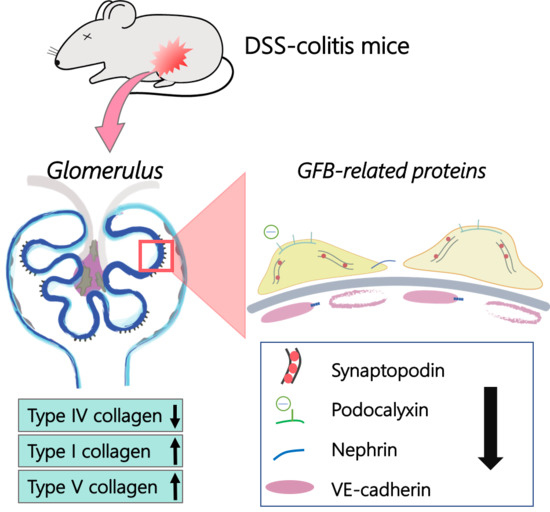
2.3. Collagen Changes in Glomeruli
2.4. Changes in GFB-Related Proteins
3. Discussion
4. Materials and Methods
4.1. Laboratory Animals
4.2. Induction of DSS-Colitis in Mice
4.3. Disease Activity Index (DAI)
4.4. Histological Investigation
4.5. Immunofluorescence Staining and Confocal Imaging
4.6. Renal Glomerular Isolation
4.7. Protein Isolation and Western Blot Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| GFB | Glomerular filtration barrier |
| DSS | Dextran sulfate sodium |
| PAS | Periodic acid-Schiff |
| DAI | Disease activity index |
| GBM | Glomerular basement membrane |
| ECM | Extracellular matrix |
References
- Sartor, R.B. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn’s disease. Gastroenterol. Clin. N. Am. 1995, 24, 475–507. [Google Scholar]
- Ricart, E.; Panaccione, R.; Loftus, E.V.; Tremaine, W.J.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. Autoimmune disorders and Extraintestinal manifestations in First-degree familial and sporadic inflammatory bowel disease. Inflamm. Bowel Dis. 2004, 10, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, D.K.; Katsanos, K.H.; Kitsanou, M.; Stergiopoulou, C.; Hatzis, J.; Tsianos, E.V. Frequency of extraintestinal manifestations in patients with inflammatory bowel disease in northwest Greece and review of the literature. Dig. Liver Dis. 2002, 34, 781–786. [Google Scholar] [CrossRef]
- Ambruzs, J.M.; Walker, P.D.; Larsen, C.P. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin. J. Am. Soc. Nephrol. 2014, 9, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Ambruzs, J.M.; Larsen, C.P. Renal Manifestations of Inflammatory Bowel Disease. Rheum. Dis. Clin. N. Am. 2018, 44, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Rabin, B.S.; Rogers, S. Pathologic changes in the liver and kidney produced by immunization with intestinal antigens. Am. J. Pathol. 1972, 84, 201–210. [Google Scholar]
- Kreisel, W.; Wolf, L.M.; Grotz, W.; Grieshaber, M. Renal tubular damage: An extraintestinal manifestation of chronic inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 1996, 8, 461–468. [Google Scholar]
- Khosroshahi, H.T.; Shoja, M.M. Tubulointerstitial disease and ulcerative colitis. Nephrol. Dial. Transplant. 2006, 21, 2340. [Google Scholar] [CrossRef]
- Corica, D.; Romano, C. Renal Involvement in Inflammatory Bowel Diseases. J. Crohns Colitis 2016, 10, 226–235. [Google Scholar] [CrossRef]
- Fraser, J.S.; Muller, A.F.; Smith, D.J.; Newman, D.J.; Lamb, E.J. Renal tubular injury is present in acute inflammatory bowel disease prior to the introduction of drug therapy. Aliment. Pharmacol. Ther. 2001, 15, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Tokuyama, H.; Wakino, S.; Konishi, K.; Hashiguchi, A.; Hayashi, K.; Itoh, H. Acute interstitial nephritis associated with ulcerative colitis. Clin. Exp. Nephrol. 2010, 14, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate sodium (DSS)-induced Colitis in mice. Trends Pharmacol. Sci. 2014, 104. [Google Scholar] [CrossRef]
- de Lange, K.M.; Barrett, J.C. Understanding inflammatory bowel disease via immunogenetics. J. Autoimmun. 2015, 64, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranganathan, P.; Jayakumar, C.; Santhakumar, M.; Ramesh, G. Netrin-1 regulates colon-kidney cross talk through suppression of IL-6 function in a mouse model of DSS-colitis. Am. J. Physiol. Renal Physiol. 2013, 304, 1187–1197. [Google Scholar] [CrossRef]
- Ranganathan, P.; Jayakumar, C.; Manicassamy, S.; Ramesh, G. CXCR2 knockout mice are protected against DSS-colitis-induced acute kidney injury and inflammation. Am. J. Physiol. Renal Physiol. 2013, 305, 1422–1427. [Google Scholar] [CrossRef]
- Meyer, T.W. Tubular injury in glomerular disease. Kidney Int. 2003, 63, 774–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriz, W.; LeHir, M. Pathways to nephron loss starting from glomerular diseases-Insights from animal models. Kidney Int. 2005, 67, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Lennon, R.; Randles, M.J.; Humphries, M.J. The Importance of Podocyte Adhesion for a Healthy Glomerulus. Front. Endocrinol. 2014, 5, 160. [Google Scholar] [CrossRef] [PubMed]
- Arif, E.; Nihalani, D. Glomerular Filtration Barrier Assembly: An insight. Postdoc. J. 2013, 1, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.P.; Quaggin, S.E. The cell biology of renal filtration. J. Cell Biol. 2015, 209, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Miner, J.H. Glomerular basement membrane composition and the filtration barrier. Pediatr. Nephrol. 2011, 26, 1413–1417. [Google Scholar] [CrossRef] [Green Version]
- Byron, A.; Randles, M.J.; Humphries, J.D.; Mironov, A.; Hamidi, H.; Harris, S.; Mathieson, P.W.; Saleem, M.A.; Satchell, S.C.; Zent, R.; et al. Glomerular Cell Cross-Talk Influences Composition and Assembly of Extracellular Matrix. J. Am. Soc. Nephrol. 2014, 25, 953–966. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.M.; Miner, J.H. Glomerular basement membrane and related glomerular disease. Transl. Res. 2012, 160, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Chew, C.; Lennon, R. Basement Membrane Defects in Genetic Kidney Diseases. Front. Pediatr. 2018, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.H.; Murasawa, Y.; Qi, P.; Nishimura, Y.; Wang, P.C. Type V collagen fibrils in mouse metanephroi. Biochem. Biophys. Res. Commun. 2013, 441, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Genovese, F.; Manresa, A.A.; Leeming, D.; Karsdal, M.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis. Fibrogenes. Tissue Repair 2014, 7, 4. [Google Scholar] [CrossRef]
- Levine, J.S.; Burakoff, R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol. Hepatol. 2011, 7, 235–241. [Google Scholar]
- Da Silva, A.P.; Pollett, A.; Rittling, S.R.; Denhardt, D.T.; Sodek, J.; Zohar, R. Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-α expression and non-programmed cell death. J. Cell Physiol. 2006, 208, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, E.A.; Dhaun, N.; Dear, J.W.; Webb, D.J. Measurement of renal function in patients with chronic kidney disease. Br. J. Clin. Pharmacol. 2013, 76, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Emamian, S.A.; Nielsen, M.B.; Pedersen, J.F.; Ytte, L. Kidney dimensions at sonography: Correlation with age, sex, and habitus in 665 adult volunteers. Am. J. Roentgenol. 1993, 160, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, D.; Andreassen, B.U.; Heegaard, N.H.H.; Klinge, L.G.; Walsted, A.M.; Neland, M.; Nielsen, R.G.; Wittenhagen, P. Pediatric Inflammatory Bowel Diseases: Should We Be Looking for Kidney Abnormalities? Inflamm. Bowel Dis. 2018, 24, 2599–2605. [Google Scholar] [CrossRef]
- Kashgarian, M.; Sterze, B. The pathobiology of the mesangium. Kidney Int. 1992, 41, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Duffield, J.S. Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Investig. 2014, 124, 2299–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miner, J.H. Renal basement membrane components. Kidney Int. 1999, 56, 2016–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, H.; Li, Y.; Li, H.; Chi, Y.; Zhuang, M.; Zhang, T.; Liu, M.; Nie, L. Matrix metalloproteinase 12 modulates high-fat-diet induced glomerular fibrogenesis and inflammation in a mouse model of obesity. Sci. Rep. 2016, 6, 20171. [Google Scholar] [CrossRef] [Green Version]
- Tamsma, J.T.; van den Born, J.; Bruijn, J.A.; Assmann, K.J.; Weening, J.J.; Berden, J.H.; Wieslander, J.; Schrama, E.; Hermans, J.; Veerkamp, J.H. Expression of glomerular extracellular matrix components in human diabetic nephropathy: Decrease of heparan sulphate in the glomerular basement membrane. Diabetologia 1994, 37, 313–320. [Google Scholar] [CrossRef]
- Morita, M.; Uchigata, Y.; Hanai, K.; Ogawa, Y.; Iwamoto, Y. Association of urinary type IV collagen with GFR decline in young patients with type 1 diabetes. Am. J. Kidney Dis. 2011, 58, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Tohda, M.; Takemura, T.; Akano, N.; Matsubara, K.; Ooshima, A.; Maki, S. Distribution of type I collagen in human kidney diseases in comparison with type III collagen. J. Pathol. 1990, 162, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, H.; Kitamura, H.; Matsuo, N.; Tatsukawa, S.; Ishikawa, K.; Okamoto, O.; Fujikura, Y.; Fujiwara, S.; Yoshioka, H. Transient expression of mouse Pro-α3(V) collagen gene (Col5a3) in wound healing. Connect. Tissue Res. 2012, 5, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Hasegawa, T.; Minamoto, T.; Oda, Y.; Inui, K.; Tayama, H.; Nakao, N.; Nakamoto, Y.; Ideura, T.; Yoshimura, A. Collagenofibrotic glomerulopathy with a widespread expression of type-v collagen. Virchows Arch. 2003, 442, 163–168. [Google Scholar] [PubMed]
- Murasawa, Y.; Hayashi, T.; Wang, P.C. The role of type V collagen fibril as an ECM that induces the motility of glomerular endothelial cells. Exp. Cell Res. 2008, 314, 3638–3653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekulic, M.; Pichler, S. A compendium of urinary biomarkers indicative of glomerular podocytopathy. Pathol. Res. Int. 2013, 2013, 782395. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.K.; Kim, S.J.; Kim, H.Y. Urine synaptopodin excretion is an important marker of glomerular disease progression. Korean J. Intern Med. 2016, 31, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.S.; McNagny, K.M. The role of podocalyxin in health and disease. J. Am. Soc. Nephrol. 2009, 20, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Greka, A.; Mundel, P. Cell biology and pathology of podocytes. Annu. Rev. Physiol. 2012, 74, 299–323. [Google Scholar] [CrossRef]
- Spagnuolo, R.; Corada, M.; Orsenigo, F.; Zanetta, L.; Deuschle, U.; Sandy, P.; Schneider, C.; Drake, C.J.; Breviario, F.; Dejana, E. Gas1 is induced by VE-cadherin and vascular endothelial growth factor and inhibits endothelial cell apoptosis. Blood 2004, 103, 3005–3012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannotta, M.; Trani, M.; Dejana, E. VE-Cadherin and endothelial Adherens Junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef]
- Hernandez, N.M.; Casselbrant, A.; Joshi, M.; Johansson, B.R.; Sumitran-Holgersson, S. Antibodies to kidney endothelial cells contribute to a “leaky” glomerular barrier in patients with chronic kidney diseases. Am. J. Physiol. Renal Physiol. 2012, 302, F884–F894. [Google Scholar] [CrossRef] [Green Version]
- Murthy, S.N.; Cooper, H.S.; Shim, H.; Shah, R.S.; Ibrahim, S.A.; Sedergran, D.J. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporine. Dig. Dis. Sci. 1993, 38, 1722–1734. [Google Scholar] [CrossRef]
- McManus, J.F. The Periodic Acid Routine Applied to the Kidney. Am. J. Pathol. 1948, 24, 643–653. [Google Scholar] [PubMed]
- Cohen, A.H. Masson’s trichrome stain in the evaluation of renal biopsies. An appraisal. Am. J. Clin. Pathol. 1976, 65, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Hsu, H.H.; Wang, P.C. Detection of initial angiogenesis from dorsal aorta into metanephroi and elucidation of its role in kidney development. Regener. Ther. 2016, 4, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Nagao, T.; Suzuki, K.; Utsunomiya, K.; Matsumura, M.; Saiga, K.; Wang, P.C.; Minamitani, H.; Aratani, Y.; Nakayama, T.; Suzuki, K. Direct activation of glomerular endothelial cells by anti-moesin activity of anti-myeloperoxidase antibody. Nephrol. Dial. Transplant. 2011, 26, 2752–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-J.; Wang, P.-C.; Huang, T.-C.; Taniguchi, A. Change in Renal Glomerular Collagens and Glomerular Filtration Barrier-Related Proteins in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. Int. J. Mol. Sci. 2019, 20, 1458. https://doi.org/10.3390/ijms20061458
Chang C-J, Wang P-C, Huang T-C, Taniguchi A. Change in Renal Glomerular Collagens and Glomerular Filtration Barrier-Related Proteins in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. International Journal of Molecular Sciences. 2019; 20(6):1458. https://doi.org/10.3390/ijms20061458
Chicago/Turabian StyleChang, Chia-Jung, Pi-Chao Wang, Tzou-Chi Huang, and Akiyoshi Taniguchi. 2019. "Change in Renal Glomerular Collagens and Glomerular Filtration Barrier-Related Proteins in a Dextran Sulfate Sodium-Induced Colitis Mouse Model" International Journal of Molecular Sciences 20, no. 6: 1458. https://doi.org/10.3390/ijms20061458
APA StyleChang, C. -J., Wang, P. -C., Huang, T. -C., & Taniguchi, A. (2019). Change in Renal Glomerular Collagens and Glomerular Filtration Barrier-Related Proteins in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. International Journal of Molecular Sciences, 20(6), 1458. https://doi.org/10.3390/ijms20061458