NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases
Abstract
:1. Inflammation in Cardiovascular Diseases
1.1. Inflammation and Atherosclerosis
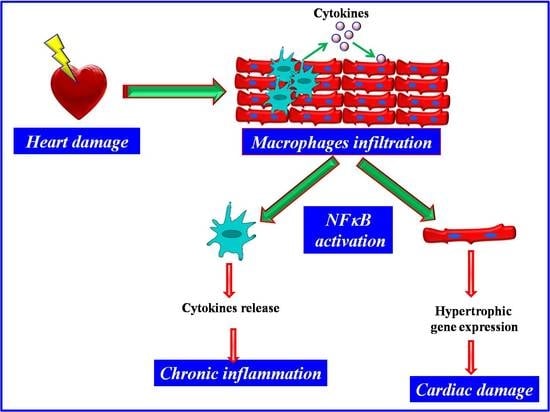
1.2. The Role of Inflammation in Myocardial Infarct and Heart Failure
1.3. Inflammation and Cardiovascular Risk
2. Targeting Inflammatory Signaling in Cardiovascular Diseases
2.1. The Modulation of Inflammatory Proteins Expression
2.2. The Therapeutic Monoclonal Antibodies
3. Molecular Mechanisms Involved in Inflammatory Responses: the Role of NFκB
4. NFκB in Cardiovascular Diseases
5. Targeting NFκB in the Failing Heart: the Role of GRKs
5.1. The RH Domain of GRK5
5.2. Synthetic Inhibitors of GRK2
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Briasoulis, A.; Androulakis, E.; Christophides, T.; Tousoulis, D. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail. Rev. 2016, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Dick, S.A.; Epelman, S. Chronic heart failure and inflammation: What do we really know? Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef]
- Mehta, J.L.; Pothineni, N.V. Inflammation in heart failure: The holy grail? Hypertension 2016, 68, 27–29. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Danesh, J.; Collins, R.; Appleby, P.; Peto, R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. JAMA 1998, 279, 1477–1482. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Charles-Schoeman, C. Cardiovascular disease and rheumatoid arthritis: An update. Curr. Rheumatol. Rep. 2012, 14, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, M.; Manabe, I.; Chester, A.; Aikawa, E. Cardiovascular inflammation. Int. J. Inflam. 2012, 2012, 904608. [Google Scholar] [CrossRef] [PubMed]
- Willerson, J.T.; Ridker, P.M. Inflammation as a cardiovascular risk factor. Circulation 2004, 109, II2–II10. [Google Scholar] [CrossRef] [PubMed]
- Packard, R.R.; Lichtman, A.H.; Libby, P. Innate and adaptive immunity in atherosclerosis. Semin. Immunopathol. 2009, 31, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Mach, F. The role of chemokines in atherosclerosis. Curr. Atheroscler. Rep. 2001, 3, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Munro, J.M. Endothelial-leukocyte adhesive interactions in inflammatory diseases. Eur. Heart J. 1993, 14, 72–77. [Google Scholar]
- Hansson, G.K.; Libby, P.; Schonbeck, U.; Yan, Z.Q. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002, 91, 281–291. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Brasier, A.R.; Recinos, A., 3rd; Eledrisi, M.S. Vascular inflammation and the renin-angiotensin system. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, M.E.; Gonzalez, W.; Nicoletti, A.; Savoie, F.; Arnal, J.F.; Michel, J.B. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 645–651. [Google Scholar] [CrossRef]
- Deng, B.; Fang, F.; Yang, T.; Yu, Z.; Zhang, B.; Xie, X. Ghrelin inhibits AngII -induced expression of TNF-alpha, IL-8, MCP-1 in human umbilical vein endothelial cells. Int. J. Clin. Exp. Med. 2015, 8, 579–588. [Google Scholar]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Bohm, M.; Nickenig, G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef]
- Tieu, B.C.; Lee, C.; Sun, H.; Lejeune, W.; Recinos, A., 3rd; Ju, X.; Spratt, H.; Guo, D.C.; Milewicz, D.; Tilton, R.G.; et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J. Clin. Investig. 2009, 119, 3637–3651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liuzzo, G.; Biasucci, L.M.; Gallimore, J.R.; Grillo, R.L.; Rebuzzi, A.G.; Pepys, M.B.; Maseri, A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N. Engl. J. Med. 1994, 331, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, L.M.; Vitelli, A.; Liuzzo, G.; Altamura, S.; Caligiuri, G.; Monaco, C.; Rebuzzi, A.G.; Ciliberto, G.; Maseri, A. Elevated levels of interleukin-6 in unstable angina. Circulation 1996, 94, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Frieler, R.A.; Mortensen, R.M. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation 2015, 131, 1019–1030. [Google Scholar] [CrossRef]
- Nian, M.; Lee, P.; Khaper, N.; Liu, P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 2004, 94, 1543–1553. [Google Scholar] [CrossRef]
- Mann, D.L. Stress-activated cytokines and the heart: From adaptation to maladaptation. Annu. Rev. Physiol. 2003, 65, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Deten, A.; Volz, H.C.; Briest, W.; Zimmer, H.G. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc. Res. 2002, 55, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.; Liu, L.; Kelley, J.; Kao, R.; Williams, D.; Li, C. Toll-like receptors: New players in myocardial ischemia/reperfusion injury. Antioxid. Redox Signal. 2011, 15, 1875–1893. [Google Scholar] [CrossRef] [PubMed]
- MacEwan, D.J. TNF receptor subtype signalling: Differences and cellular consequences. Cell Signal. 2002, 14, 477–492. [Google Scholar] [CrossRef]
- Machida, Y.; Kubota, T.; Kawamura, N.; Funakoshi, H.; Ide, T.; Utsumi, H.; Li, Y.Y.; Feldman, A.M.; Tsutsui, H.; Shimokawa, H.; et al. Overexpression of tumor necrosis factor-alpha increases production of hydroxyl radical in murine myocardium. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H449–H455. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.T.; Wu, C.K.; Lee, J.K.; Chang, S.N.; Kuo, Y.M.; Wang, Y.C.; Lai, L.P.; Chiang, F.T.; Hwang, J.J.; Lin, J.L. TNF-alpha down-regulates sarcoplasmic reticulum Ca(2)(+) ATPase expression and leads to left ventricular diastolic dysfunction through binding of NF-kappaB to promoter response element. Cardiovasc. Res. 2015, 105, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Fushimi, K.; Kouchi, H.; Mihara, K.; Miyazaki, M.; Ohe, T.; Namba, M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation 1998, 98, 794–799. [Google Scholar] [CrossRef]
- Suematsu, N.; Tsutsui, H.; Wen, J.; Kang, D.; Ikeuchi, M.; Ide, T.; Hayashidani, S.; Shiomi, T.; Kubota, T.; Hamasaki, N.; et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 2003, 107, 1418–1423. [Google Scholar] [CrossRef]
- Yokoyama, T.; Vaca, L.; Rossen, R.D.; Durante, W.; Hazarika, P.; Mann, D.L. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J. Clin. Investig. 1993, 92, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, J.I.; Kim, K.H.; Natterson, P.D.; Lawrence, T.; Yang, P.; Weiss, J.N. Effects of TNF-alpha on [Ca2+]i and contractility in isolated adult rabbit ventricular myocytes. Am. J. Physiol. 1996, 271, H1449–H1455. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Panas, D.L.; Catena, R.; Moncada, S.; Olley, P.M.; Lopaschuk, G.D. The role of nitric oxide in cardiac depression induced by interleukin-1 beta and tumour necrosis factor-alpha. Br. J. Pharmacol. 1995, 114, 27–34. [Google Scholar] [CrossRef]
- Tatsumi, T.; Matoba, S.; Kawahara, A.; Keira, N.; Shiraishi, J.; Akashi, K.; Kobara, M.; Tanaka, T.; Katamura, M.; Nakagawa, C.; et al. Cytokine-induced nitric oxide production inhibits mitochondrial energy production and impairs contractile function in rat cardiac myocytes. J. Am. Coll. Cardiol. 2000, 35, 1338–1346. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Raleigh, J.M.; Abbate, A. Targeting interleukin-1 in heart failure and inflammatory heart disease. Curr. Heart Fail. Rep. 2015, 12, 33–41. [Google Scholar] [CrossRef]
- Gambardella, J.; Santulli, G. Integrating diet and inflammation to calculate cardiovascular risk. Atherosclerosis 2016, 253, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Gullestad, L.; Aukrust, P. Review of trials in chronic heart failure showing broad-spectrum anti-inflammatory approaches. Am. J. Cardiol. 2005, 95, 17–23. [Google Scholar] [CrossRef]
- Aukrust, P.; Yndestad, A.; Damas, J.K.; Gullestad, L. Inflammation and chronic heart failure-potential therapeutic role of intravenous immunoglobulin. Autoimmun. Rev. 2004, 3, 221–227. [Google Scholar] [CrossRef]
- Mann, D.L. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ. Res. 2002, 91, 988–998. [Google Scholar] [CrossRef]
- Yndestad, A.; Damas, J.K.; Oie, E.; Ueland, T.; Gullestad, L.; Aukrust, P. Role of inflammation in the progression of heart failure. Curr. Cardiol. Rep. 2007, 9, 236–241. [Google Scholar] [CrossRef]
- Dhawan, S.S.; Quyyumi, A.A. Rheumatoid arthritis and cardiovascular disease. Curr. Atheroscler. Rep. 2008, 10, 128–133. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef]
- Choi, H.K.; Hernan, M.A.; Seeger, J.D.; Robins, J.M.; Wolfe, F. Methotrexate and mortality in patients with rheumatoid arthritis: A prospective study. Lancet 2002, 359, 1173–1177. [Google Scholar] [CrossRef]
- Krishnan, E.; Lingala, V.B.; Singh, G. Declines in mortality from acute myocardial infarction in successive incidence and birth cohorts of patients with rheumatoid arthritis. Circulation 2004, 110, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, L.; Boffini, N.; Cagnotto, G.; Inverardi, F.; Grosso, V.; Caporali, R. Atherosclerosis and rheumatoid arthritis: More than a simple association. Mediat. Inflamm. 2012, 2012, 147354. [Google Scholar] [CrossRef]
- Ohta, H.; Wada, H.; Niwa, T.; Kirii, H.; Iwamoto, N.; Fujii, H.; Saito, K.; Sekikawa, K.; Seishima, M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis 2005, 180, 11–17. [Google Scholar] [CrossRef]
- Devlin, C.M.; Kuriakose, G.; Hirsch, E.; Tabas, I. Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proc. Natl. Acad. Sci. USA 2002, 99, 6280–6285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charo, I.F.; Taub, R. Anti-inflammatory therapeutics for the treatment of atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serruys, P.W.; Garcia-Garcia, H.M.; Buszman, P.; Erne, P.; Verheye, S.; Aschermann, M.; Duckers, H.; Bleie, O.; Dudek, D.; Botker, H.E.; et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 2008, 118, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; McMurray, J.J.; Klug, E.; Small, R.; Schumi, J.; Choi, J.; Cooper, J.; Scott, R.; Lewis, E.F.; L’Allier, P.L.; et al. Aggressive reduction of inflammation stops events trial, i., effects of succinobucol (AGI-1067) after an acute coronary syndrome: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 1761–1768. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Clearfield, M.; Downs, J.R.; Weis, S.E.; Miles, J.S.; Gotto, A.M., Jr. Air force/Texas coronary atherosclerosis prevention study, i., measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N. Engl. J. Med. 2001, 344, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Siennicka, A.; Kaczmarczyk, M.; Kolodziej, B.; Naruszewicz, M. Simvastatin modulates TNFalpha-induced adhesion molecules expression in human endothelial cells. Life Sci. 2004, 75, 1287–1302. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, M.; Rabkin, E.; Sugiyama, S.; Voglic, S.J.; Fukumoto, Y.; Furukawa, Y.; Shiomi, M.; Schoen, F.J.; Libby, P. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 2001, 103, 276–283. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Margaritis, M.; Lee, R.; Channon, K.; Antoniades, C. Statins as anti-inflammatory agents in atherogenesis: Molecular mechanisms and lessons from the recent clinical trials. Curr. Pharm. Des. 2012, 18, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Pliquett, R.U.; Cornish, K.G.; Peuler, J.D.; Zucker, I.H. Simvastatin normalizes autonomic neural control in experimental heart failure. Circulation 2003, 107, 2493–2498. [Google Scholar] [CrossRef]
- Node, K.; Fujita, M.; Kitakaze, M.; Hori, M.; Liao, J.K. Short-term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathy. Circulation 2003, 108, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Matsumori, A.; Yamada, T.; Shioi, T.; Wang, W.; Ono, K.; Nishio, R.; Okada, M.; Sasayama, S. Pimobendan inhibits the production of proinflammatory cytokines and gene expression of inducible nitric oxide synthase in a murine model of viral myocarditis. J. Am. Coll. Cardiol. 1999, 33, 1400–1407. [Google Scholar] [CrossRef] [Green Version]
- Matsumori, A.; Nunokawa, Y.; Sasayama, S. Pimobendan inhibits the activation of transcription factor NF-kappaB: A mechanism which explains its inhibition of cytokine production and inducible nitric oxide synthase. Life Sci. 2000, 67, 2513–2519. [Google Scholar] [CrossRef]
- Effects of Pimobendan on Chronic Heart Failure Study. Effects of pimobendan on adverse cardiac events and physical activities in patients with mild to moderate chronic heart failure: The effects of pimobendan on chronic heart failure study (EPOCH study). Circ. J. 2002, 66, 149–157. [Google Scholar]
- Matsumori, A.; Ono, K.; Nishio, R.; Nose, Y.; Sasayama, S. Amiodarone inhibits production of tumor necrosis factor-alpha by human mononuclear cells: A possible mechanism for its effect in heart failure. Circulation 1997, 96, 1386–1389. [Google Scholar] [CrossRef]
- Ito, H.; Ono, K.; Nishio, R.; Sasayama, S.; Matsumori, A. Amiodarone inhibits interleukin 6 production and attenuates myocardial injury induced by viral myocarditis in mice. Cytokine 2002, 17, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Chenevard, R.; Hurlimann, D.; Bechir, M.; Enseleit, F.; Spieker, L.; Hermann, M.; Riesen, W.; Gay, S.; Gay, R.E.; Neidhart, M.; et al. Selective COX-2 inhibition improves endothelial function in coronary artery disease. Circulation 2003, 107, 405–409. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Price, D.T.; Gokce, N.; Eberhardt, R.T.; Duffy, S.J.; Holbrook, M.; Maxwell, C.; Palmisano, J.; Keaney, J.F., Jr.; Morrow, J.D.; et al. Short- and long-term COX-2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension 2003, 42, 310–315. [Google Scholar] [CrossRef]
- Fliser, D.; Buchholz, K.; Haller, H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation 2004, 110, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Loppnow, H.; Libby, P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J. Clin. Investig. 1990, 85, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Libby, P.; MacFadyen, J.G.; Thuren, T.; Ballantyne, C.; Fonseca, F.; Koenig, W.; Shimokawa, H.; Everett, B.M.; Glynn, R.J. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: Analyses from the canakinumab anti-inflammatory thrombosis outcomes study (CANTOS). Eur. Heart J. 2018, 39, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Kleveland, O.; Kunszt, G.; Bratlie, M.; Ueland, T.; Broch, K.; Holte, E.; Michelsen, A.E.; Bendz, B.; Amundsen, B.H.; Espevik, T.; et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: A double-blind, randomized, placebo-controlled phase 2 trial. Eur. Heart J. 2016, 37, 2406–2413. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation 2003, 107, 3133–3140. [Google Scholar] [PubMed]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R. The NF-kappaB regulatory network. Cardiovasc. Toxicol. 2006, 6, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. 2007, 8, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. The Rel/NF-kappaB signal transduction pathway: Introduction. Oncogene 1999, 18, 6842–6844. [Google Scholar] [CrossRef]
- Tian, B.; Brasier, A.R. Identification of a nuclear factor kappa B-dependent gene network. Recent Prog. Horm. Res. 2003, 58, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The noncanonical NF-kappaB pathway. Immunol. Rev. 2012, 246, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Liu, P.P.; Mann, D.L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 2015, 15, 117–129. [Google Scholar] [CrossRef]
- Baldwin, A.S., Jr. Series introduction: The transcription factor NF-kappaB and human disease. J. Clin. Investig. 2001, 107, 3–6. [Google Scholar] [CrossRef]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-kappaB: Its role in health and disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Role of the NF-kappaB pathway in the pathogenesis of human disease states. Curr. Mol. Med. 2001, 1, 287–296. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Santulli, G.; Franco, A.; Cipolletta, E.; Napolitano, L.; Gambardella, J.; Gomez-Monterrey, I.; Campiglia, P.; Trimarco, B.; Iaccarino, G.; et al. Integrating GRK2 and NFkappaB in the Pathophysiology of Cardiac Hypertrophy. J. Cardiovasc. Transl. Res. 2015, 8, 493–502. [Google Scholar] [CrossRef]
- Sorriento, D.; Santulli, G.; Fusco, A.; Anastasio, A.; Trimarco, B.; Iaccarino, G. Intracardiac injection of AdGRK5-NT reduces left ventricular hypertrophy by inhibiting NF-kappaB-dependent hypertrophic gene expression. Hypertension 2010, 56, 696–704. [Google Scholar] [CrossRef]
- Ritchie, M.E. Nuclear factor-kappaB is selectively and markedly activated in humans with unstable angina pectoris. Circulation 1998, 98, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, B.; Freeman, G.L. Induction of nuclear factor kappaB and activation protein 1 in postischemic myocardium. FEBS Lett. 1997, 401, 30–34. [Google Scholar] [CrossRef]
- Li, C.; Kao, R.L.; Ha, T.; Kelley, J.; Browder, I.W.; Williams, D.L. Early activation of IKKbeta during in vivo myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1264–H1271. [Google Scholar] [CrossRef]
- Li, C.; Browder, W.; Kao, R.L. Early activation of transcription factor NF-kappaB during ischemia in perfused rat heart. Am. J. Physiol. 1999, 276, H543–H552. [Google Scholar]
- Valen, G.; Hansson, G.K.; Dumitrescu, A.; Vaage, J. Unstable angina activates myocardial heat shock protein 72, endothelial nitric oxide synthase, and transcription factors NFkappaB and AP-1. Cardiovasc. Res. 2000, 47, 49–56. [Google Scholar] [CrossRef]
- Valen, G. Signal transduction through nuclear factor kappa B in ischemia-reperfusion and heart failure. Basic Res. Cardiol. 2004, 99, 1–7. [Google Scholar] [CrossRef]
- Siednienko, J.; Jankowska, E.A.; Banasiak, W.; Gorczyca, W.A.; Ponikowski, P. Nuclear factor-kappaB activity in peripheral blood mononuclear cells in cachectic and non-cachectic patients with chronic heart failure. Int. J. Cardiol. 2007, 122, 111–116. [Google Scholar] [CrossRef]
- Frantz, S.; Stoerk, S.; Ok, S.; Wagner, H.; Angermann, C.E.; Ertl, G.; Bauersachs, J. Effect of chronic heart failure on nuclear factor kappa B in peripheral leukocytes. Am. J. Cardiol. 2004, 94, 671–673. [Google Scholar] [CrossRef]
- Shaw, J.; Zhang, T.; Rzeszutek, M.; Yurkova, N.; Baetz, D.; Davie, J.R.; Kirshenbaum, L.A. Transcriptional silencing of the death gene BNIP3 by cooperative action of NF-kappaB and histone deacetylase 1 in ventricular myocytes. Circ. Res. 2006, 99, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.W.; Shaw, J.A.; Kirshenbaum, L.A. Multiple facets of NF-kappaB in the heart: To be or not to NF-kappaB. Circ. Res. 2011, 108, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.J.; Schips, T.G.; Wietelmann, A.; Kruger, M.; Brunner, C.; Sauter, M.; Klingel, K.; Bottger, T.; Braun, T.; Wirth, T. Cardiomyocyte-specific IkappaB kinase (IKK)/NF-kappaB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc. Natl. Acad. Sci. USA 2012, 109, 11794–11799. [Google Scholar] [CrossRef]
- Hamid, T.; Guo, S.Z.; Kingery, J.R.; Xiang, X.; Dawn, B.; Prabhu, S.D. Cardiomyocyte NF-kappaB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc. Res. 2011, 89, 129–138. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Auger-Messier, M.; Molkentin, J.D. Interaction between NFkappaB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ. Res. 2012, 110, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Young, D.; Maitra, R.K.; Gupta, A.; Popovic, Z.B.; Yong, S.L.; Mahajan, A.; Wang, Q.; Sen, S. Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. J. Mol. Biol. 2008, 375, 637–649. [Google Scholar] [CrossRef]
- Donnarumma, E.; Ali, M.J.; Rushing, A.M.; Scarborough, A.L.; Bradley, J.M.; Organ, C.L.; Islam, K.N.; Polhemus, D.J.; Evangelista, S.; Cirino, G.; et al. Zofenopril protects against myocardial ischemia-reperfusion injury by increasing nitric oxide and hydrogen sulfide bioavailability. J. Am. Heart. Assoc. 2016, 5, e003531. [Google Scholar] [CrossRef] [PubMed]
- Fratta Pasini, A.; Garbin, U.; Nava, M.C.; Stranieri, C.; Davoli, A.; Sawamura, T.; Lo Cascio, V.; Cominacini, L. Nebivolol decreases oxidative stress in essential hypertensive patients and increases nitric oxide by reducing its oxidative inactivation. J. Hypertens. 2005, 23, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.S.; Babbar, R.; Kansal, N.; Agarwal, S.K.; Ray, P.C. Antihypertensive and antioxidant action of amlodipine and vitamin C in patients of essential hypertension. J. Clin. Biochem. Nutr. 2007, 40, 141–147. [Google Scholar] [CrossRef]
- Yao, E.H.; Fukuda, N.; Matsumoto, T.; Katakawa, M.; Yamamoto, C.; Han, Y.; Ueno, T.; Kobayashi, N.; Matsumoto, K. Effects of the antioxidative beta-blocker celiprolol on endothelial progenitor cells in hypertensive rats. Am. J. Hypertens. 2008, 21, 1062–1068. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar] [CrossRef]
- Vafadari, R.; Kraaijeveld, R.; Weimar, W.; Baan, C.C. Tacrolimus inhibits NF-kappaB activation in peripheral human T cells. PLoS ONE 2013, 8, e60784. [Google Scholar] [CrossRef]
- Mishra, K.P.; Chanda, S.; Karan, D.; Ganju, L.; Sawhney, R.C. Effect of Seabuckthorn (Hippophae rhamnoides) flavone on immune system: An in-vitro approach. Phytother. Res. 2008, 22, 1490–1495. [Google Scholar] [CrossRef]
- Kasinski, A.L.; Du, Y.; Thomas, S.L.; Zhao, J.; Sun, S.Y.; Khuri, F.R.; Wang, C.Y.; Shoji, M.; Sun, A.; Snyder, J.P.; et al. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol. Pharmacol. 2008, 74, 654–661. [Google Scholar] [CrossRef]
- Pujols, L.; Fernandez-Bertolin, L.; Fuentes-Prado, M.; Alobid, I.; Roca-Ferrer, J.; Agell, N.; Mullol, J.; Picado, C. Proteasome inhibition reduces proliferation, collagen expression, and inflammatory cytokine production in nasal mucosa and polyp fibroblasts. J. Pharmacol. Exp. Ther. 2012, 343, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Momose, I.; Iijima, M.; Kawada, M.; Ikeda, D. A new proteasome inhibitor, TP-110, induces apoptosis in human prostate cancer PC-3 cells. Biosci. Biotechnol. Biochem. 2007, 71, 1036–1043. [Google Scholar] [CrossRef]
- Nam, K.N.; Son, M.S.; Park, J.H.; Lee, E.H. Shikonins attenuate microglial inflammatory responses by inhibition of ERK, Akt, and NF-kappaB: Neuroprotective implications. Neuropharmacology 2008, 55, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, R.; Naka, T.; Tsutsui, H.; Fujimoto, M.; Kimura, A.; Abe, T.; Seki, E.; Sato, S.; Takeuchi, O.; Takeda, K.; et al. SOCS-1 participates in negative regulation of LPS responses. Immunity 2002, 17, 677–687. [Google Scholar] [CrossRef]
- Iwamoto, S.; Kumamoto, T.; Azuma, E.; Hirayama, M.; Ito, M.; Amano, K.; Ido, M.; Komada, Y. The effect of azithromycin on the maturation and function of murine bone marrow-derived dendritic cells. Clin. Exp. Immunol. 2011, 166, 385–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradeep, C.R.; Kuttan, G. Piperine is a potent inhibitor of nuclear factor-kappaB (NF-kappaB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. Int. Immunopharmacol. 2004, 4, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, S.L.; Blaxall, B.C. G protein coupled receptor kinases as therapeutic targets in cardiovascular disease. Circ. Res. 2011, 109, 309–319. [Google Scholar] [CrossRef]
- Sorriento, D.; Ciccarelli, M.; Cipolletta, E.; Trimarco, B.; Iaccarino, G. “Freeze, don’t move”: How to arrest a suspect in heart failure—A review on available GRK2 inhibitors. Front. Cardiovasc. Med. 2016, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Trimarco, B.; Iaccarino, G.; Sorriento, D. Cardiac nonmyocyte cell functions and crosstalks in response to cardiotoxic drugs. Oxid. Med. Cell. Longev. 2017, 2017, 1089359. [Google Scholar] [CrossRef]
- Sorriento, D.; Ciccarelli, M.; Santulli, G.; Illario, M.; Trimarco, B.; Iaccarino, G. Trafficking GRK2: Cellular and Metabolic consequences of GRK2 subcellular localization. Transl. Med. UniSa 2014, 10, 3–7. [Google Scholar]
- Sorriento, D.; Pascale, A.V.; Finelli, R.; Carillo, A.L.; Annunziata, R.; Trimarco, B.; Iaccarino, G. Targeting mitochondria as therapeutic strategy for metabolic disorders. Sci. World J. 2014, 2014, 604685. [Google Scholar] [CrossRef]
- Mak, J.C.; Hisada, T.; Salmon, M.; Barnes, P.J.; Chung, K.F. Glucocorticoids reverse IL-1beta-induced impairment of beta-adrenoceptor-mediated relaxation and up-regulation of G-protein-coupled receptor kinases. Br. J. Pharmacol. 2002, 135, 987–996. [Google Scholar] [CrossRef]
- Lombardi, M.S.; Kavelaars, A.; Cobelens, P.M.; Schmidt, R.E.; Schedlowski, M.; Heijnen, C.J. Adjuvant arthritis induces down-regulation of G protein-coupled receptor kinases in the immune system. J. Immunol. 2001, 166, 1635–1640. [Google Scholar] [CrossRef]
- Sorriento, D.; Fusco, A.; Ciccarelli, M.; Rungi, A.; Anastasio, A.; Carillo, A.; Dorn, G.W., 2nd; Trimarco, B.; Iaccarino, G. Mitochondrial G protein coupled receptor kinase 2 regulates proinflammatory responses in macrophages. FEBS Lett. 2013, 587, 3487–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vroon, A.; Kavelaars, A.; Limmroth, V.; Lombardi, M.S.; Goebel, M.U.; Van Dam, A.M.; Caron, M.G.; Schedlowski, M.; Heijnen, C.J. G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Immunol. 2005, 174, 4400–4406. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, T.K.; Rampersad, R.R.; Esserman, D.; Rothlein, L.R.; Liu, P.; Premont, R.T.; Lefkowitz, R.J.; Lee, D.M.; Patel, D.D. Granulocyte chemotaxis and disease expression are differentially regulated by GRK subtype in an acute inflammatory arthritis model (K/BxN). Clin. Immunol. 2008, 129, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardi, M.S.; Kavelaars, A.; Schedlowski, M.; Bijlsma, J.W.; Okihara, K.L.; Van de Pol, M.; Ochsmann, S.; Pawlak, C.; Schmidt, R.E.; Heijnen, C.J. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J. 1999, 13, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Koller, S.; Abd Alla, J.; Quitterer, U. Inhibition of G-protein-coupled receptor kinase 2 (GRK2) triggers the growth-promoting mitogen-activated protein kinase (MAPK) pathway. J. Biol. Chem. 2013, 288, 7738–7755. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.; Pao, C.S.; Leonhard, K.S.; Kang, D.S.; Kratz, M.; Ley, S.C.; Benovic, J.L. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J. Biol. Chem. 2006, 281, 34159–34170. [Google Scholar] [CrossRef] [PubMed]
- Steury, M.D.; McCabe, L.R.; Parameswaran, N. G protein-coupled receptor kinases in the inflammatory response and signaling. Adv. Immunol. 2017, 136, 227–277. [Google Scholar] [PubMed]
- Patial, S.; Luo, J.; Porter, K.J.; Benovic, J.L.; Parameswaran, N. G-protein-coupled-receptor kinases mediate TNFalpha-induced NFkappaB signalling via direct interaction with and phosphorylation of IkappaBalpha. Biochem. J. 2009, 425, 169–178. [Google Scholar] [CrossRef]
- Patial, S.; Saini, Y.; Parvataneni, S.; Appledorn, D.M.; Dorn, G.W., 2nd; Lapres, J.J.; Amalfitano, A.; Senagore, P.; Parameswaran, N. Myeloid-specific GPCR kinase-2 negatively regulates NF-kappaB1p105-ERK pathway and limits endotoxemic shock in mice. J. Cell. Physiol. 2011, 226, 627–637. [Google Scholar] [CrossRef]
- Xu, F.; Sun, S.; Wang, X.; Ni, E.; Zhao, L.; Zhu, W. GRK2 mediates arginine vasopressin-induced interleukin-6 production via nuclear factor-kappab signaling neonatal rat cardiac fibroblast. Mol. Pharmacol. 2017, 92, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Ciccarelli, M.; Santulli, G.; Campanile, A.; Altobelli, G.G.; Cimini, V.; Galasso, G.; Astone, D.; Piscione, F.; Pastore, L.; et al. The G-protein-coupled receptor kinase 5 inhibits NFkappaB transcriptional activity by inducing nuclear accumulation of IkappaB alpha. Proc. Natl. Acad. Sci. USA 2008, 105, 17818–17823. [Google Scholar] [CrossRef]
- Ohba, Y.; Nakaya, M.; Watari, K.; Nagasaka, A.; Kurose, H. GRK6 phosphorylates IkappaBalpha at Ser(32)/Ser(36) and enhances TNF-alpha-induced inflammation. Biochem. Biophys. Res. Commun. 2015, 461, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Santulli, G.; Ciccarelli, M.; Maione, A.S.; Illario, M.; Trimarco, B.; Iaccarino, G. The amino-terminal domain of GRK5 inhibits cardiac hypertrophy through the regulation of calcium-calmodulin dependent transcription factors. Int. J. Mol. Sci. 2018, 19, 861. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Campanile, A.; Santulli, G.; Leggiero, E.; Pastore, L.; Trimarco, B.; Iaccarino, G. A new synthetic protein, TAT-RH, inhibits tumor growth through the regulation of NFkappaB activity. Mol. Cancer 2009, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Ciccarelli, M.; Del Giudice, C.; Fiordelisi, A.; De Rosa, M.; Sala, M.; Pacelli, R.; Campiglia, P.; Trimarco, B.; Iaccarino, G.; et al. A novel small peptide inhibitor of NFkappaB, RH10, blocks oxidative stress-dependent phenotypes in cancer. Oxid. Med. Cell. Longev. 2018, 2018, 5801807. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, R.; Lunning, M.; Mantha, S.; Devlin, S.; Hamilton, A.; Schwartz, G.; Soff, G. Romiplostim for management of chemotherapy-induced thrombocytopenia. Support. Care Cancer 2014, 22, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, E.; Campanile, A.; Santulli, G.; Sanzari, E.; Leosco, D.; Campiglia, P.; Trimarco, B.; Iaccarino, G. The G protein coupled receptor kinase 2 plays an essential role in beta-adrenergic receptor-induced insulin resistance. Cardiovasc. Res. 2009, 84, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiordelisi, A.; Iaccarino, G.; Morisco, C.; Coscioni, E.; Sorriento, D. NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 1599. https://doi.org/10.3390/ijms20071599
Fiordelisi A, Iaccarino G, Morisco C, Coscioni E, Sorriento D. NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. International Journal of Molecular Sciences. 2019; 20(7):1599. https://doi.org/10.3390/ijms20071599
Chicago/Turabian StyleFiordelisi, Antonella, Guido Iaccarino, Carmine Morisco, Enrico Coscioni, and Daniela Sorriento. 2019. "NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases" International Journal of Molecular Sciences 20, no. 7: 1599. https://doi.org/10.3390/ijms20071599
APA StyleFiordelisi, A., Iaccarino, G., Morisco, C., Coscioni, E., & Sorriento, D. (2019). NFkappaB is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. International Journal of Molecular Sciences, 20(7), 1599. https://doi.org/10.3390/ijms20071599