1. Introduction
BMPs (Bone Morphogenetic Proteins) are the key proteins mediating mesenchymal stromal cell (MSC) recruitment, differentiation, and maturation into osteoblasts [
1,
2]. Since the Food and Drug Administration (FDA) approval of recombinant human (rh) BMP-2 in 2002, it has been widely adopted clinically. rhBMP-2 delivery demonstrates consistent bone formation, but has also been associated with several local adverse side effects including inflammation, vascular permeability, seromas, hematomas, and nerve root irritation [
3,
4,
5,
6,
7,
8]. Most of the initial BMP-2 dose is released from its collagen carrier in the first several days, leaving only a small amount of residual BMP-2 to initiate differentiation of osteoprogenitors as they arrive during the first two weeks. Simply lowering the initial BMP dose does not solve the clinical problems, as the residual dose would also drop below the threshold of the necessary dose for consistent bone induction in humans.
The current first-generation clinical strategy for BMP-induced bone formation involves the traditional ligand-dependent activation of the BMP/Smad pathway, which begins when rhBMP protein binds to the receptor complex. BMP receptors (BMPRs) are the transmembrane serine threonine kinase receptors, classified as type I (BMPRI) or type II (BMPRII) based on their sequence homology [
9]. BMPRI, when activated by BMPRII upon ligand binding, phosphorylates receptor-associated Smad proteins. The activated pSmads associate with the common Smad, Smad4, and proceed to the nucleus to turn on the target gene transcription (
Figure 1) [
10,
11]. Phosphorylated Smad1/5 can be dephosphorylated by calcineurin [
12,
13].
Recently, an intracellular BMP repressor that targets BMPRI has been identified [
14]. The immunophilin FK-binding-protein-12 (FKBP12) is an intracellular BMP inhibitor and serves in preventing leaky signaling under sub-optimal ligand concentrations. FKBP12 prevents the type II receptor from phosphorylating the type I receptor, and thus prevents the association and subsequent phosphorylation of cytosolic receptor Smads [
14]. A type I receptor mutation in Fibrodysplasia Ossificans Progressiva (FOP) reduces FKBP12 binding to receptor and results in leaky BMP signaling in the absence of ligand, leading to spontaneous ossification, indicative of a potential therapeutic route for osteogenesis through targeting FKBP12 [
15].
FK506 is a lipophilic 23-member macrolide lactone isolated from
Streptomyces tsukubaensis (molecular weight of 803.5 Da). It is an immunosuppressant that is widely used in transplant patients and is typically delivered systemically (with a half-life of 8.7–11.3 h) [
16,
17]. FK506 induces its effects in part by binding to FKBP12, inhibiting calcineurin activity, and preventing T cell proliferation [
18]. Most members of the FKBP family bind to FK506 and show peptidylprolyl cis/trans isomerase (PPIase) activity. Small-sized FKBP family members contain the FK506-binding domain, while the large FKBPs also possess extra domains, such as tetratricopeptide repeat domains, and calmodulin binding and transmembrane motifs [
18].
In addition to the immunosuppressive activity, FK506 has been shown to exercise a variety of actions on bone metabolism and osteogenesis. In vitro, FK506 can enhance BMP-2 activity and promote osteogenic differentiation of various cell lines including rat MSCs, mouse pre-osteoblasts, and limb bud cells, among others [
19,
20,
21,
22,
23]. In vivo, when administered systemically in a continuous manner, FK506 causes osteopenia in mice, rats, and humans [
14,
24]. However, both bone healing and induction of ectopic mineralization have been shown to be enhanced by systemic delivery of FK506 [
25,
26,
27,
28]. Further, when administered locally in combination with BMPs (or
BMP gene therapy), FK506 enhances BMP-induced mineralization [
29]. These data present compelling utility for FK506 as an osteoinductive stimulus, but the local delivery of FK506 in a stand-alone manner has not been performed.
Our objective was to test the osteogenic activity of FK506 as a stand-alone agent both in vitro and in vivo. We hypothesized that the transient activation of BMP signaling through FK506 delivery would initiate the local osteoinductive cascade for induction of bone without adjunctive recombinant BMP or implanted MSCs. Herein, we performed in vitro dose–response studies with FK506 to induce an osteogenic response in comparison to the dose response of BMP-2 in vitro, and tested the local delivery of escalating doses of FK506 delivered locally on a collagen sponge in an ectopic mineralization assay in vivo.
3. Discussion
The need for “supra-physiologic” BMP concentrations in the clinical setting stems at least in part from the fact that human MSCs are less responsive to BMP-2 at a cellular level, and the influx of MSCs to bone healing sites is slower in humans compared to rodents or primates [
31,
32]. As a result, higher initial loading concentrations of rhBMP-2 on the collagen carrier have been required to ensure enough BMP remains at the site when cells arrive. This larger total dose (12–40 mg in humans versus μg doses in rodents for one level spine fusion) has resulted in significant local side effects (swelling, bone resorption, nerve inflammation, etc.) that were not seen in pre-clinical studies at lower BMP concentrations [
6]. These issues have resulted in an over 50% drop in usage since its peak in 2007. As such, a safe and cost-effective alternative approach to activate the BMP bone formation pathway remains of paramount clinical significance. The ligand-independent initiation of the BMP signaling pathway by FK506 has the potential to bypass these extracellular regulatory feedback mechanisms. We hypothesized that transient exposure to FK506 by local delivery in vivo would be capable of ligand-independent activation of the BMP pathway, thereby taking advantage of positive BMP feedback loops and avoiding some of the negative feedback signals.
In order to translate FK506 to a potential stand-alone local therapeutic, we started by performing a direct in vitro comparison of the dose responses of FK506 and BMP-2 to induce ALP activity in C2C12 cells. Here, we observed that an FK506 dose range from 1.5–6 µM showed a similar dose response to BMP-2 (12–50 ng·mL
−1) for inducing ALP activity in C2C12 cells. Further, we show that FK506 is able to activate both the BMP-2 and TGF-β pathways by increasing pSmad levels and subsequently by increasing the expression of early response genes. The higher levels of pSmad reflect the increased activity of receptor kinases in both pathways, while unphosphorylated levels of the Smads remained at the same level as the controls. These data are consistent with previous studies which have shown the ability of FK506 to induce BMP signaling and osteogenic differentiation in a variety of cell types, including C17 (mouse limb bud-derived cells), C2C12 (mouse myoblasts), MC3T3 (mouse pre-osteoblasts), and mesenchymal stromal cells [
19,
20,
21,
22,
23]. We further investigated the effects of FK506 on calcineurin activity and found decreased calcineurin activity in the C2C12 cells. Calcineurin has been shown to dephosphorylate pSmad1/5, and inhibition of this effect may suggest another mechanism by which FK506 could affect the BMP signaling pathway and feedback system [
13].
In addition to the direct activation of BMP signaling and osteogenic differentiation by FK506, we investigated the potential feedback control of the BMP pathway by the primary extracellular inhibitor Noggin. Noggin is a natural extracellular inhibitor of BMP activity, and can be upregulated in response to BMP signaling to provide a feedback control mechanism. Noggin exerts its function by direct binding to BMP stoichiometrically. Here we show that FK506 maintained the ability to induce ALP activity in C2C12 cells even in the presence of Noggin. The lack of Noggin-induced inhibition of FK506 confirmed ligand-independent activity and bypasses the typical feedback mechanism of BMP signaling which in part necessitates the supra-physiologic dosing used for therapeutic applications.
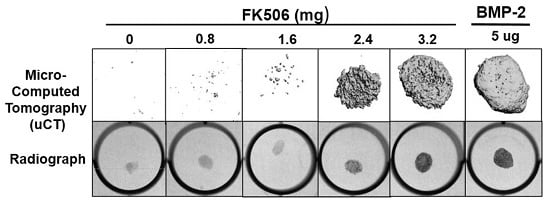
The evidence of osteogenic activity of FK506 in vitro strongly supports its potential utility in vivo. Here we show that FK506 can be delivered in vivo on a collagen sponge to induce mineralization in an ectopic site. The quantitative microCT data showed a dose-dependent response to FK506 delivery reaching a comparable level of mineralization to that of BMP-2 delivery. This was consistent with histologic observations of the mineralized tissue. These data show that FK506 can induce ligand-independent mineralization when delivered as a stand-alone agent locally on a collagen sponge.
Previous studies have not utilized stand-alone local delivery of FK506 for bone healing applications, but instead have focused on systemic or local delivery of FK506 to enhance a different osteogenic stimulus (osteogenic cell- or BMP-2-based). Systemic administration of FK506 (1 mg/kg/d (injected) or 3 mg/kg/d (oral gavage)) was shown to enhance local MSC repair of segmental defects and systemic bone formation in the setting of inflammatory-mediated bone loss. Other studies have similarly showed that systemic delivery of FK506 could enhance the healing of bone defects treated with various combinations of osteoprogenitor cells (some genetically modified) or BMP-2 [
25,
26,
27]. Systemic delivery of FK506 was also shown to enhance mineralization of a demineralized bone matrix carrier [
28]. Separate from enhancing mineralization of implanted matrix or repairing a defect, systemic FK506 can also enhance fracture healing in impaired healing scenarios like that of multi-tissue trauma. While most bone regeneration studies have utilized systemic delivery, one study showed that local delivery of a
BMP gene therapy and FK506 (1.6 mg/kg) could enhance the BMP-induced mineralization [
29]. We found that a significantly higher dose than used previously was needed for a single local delivery to induce mineralization. The dose that produced consistent mineralization comparable to BMP-2 was 3.2 mg, which would be about 12.8 mg/kg. The lowest dose we used was 0.8 mg (about 3.2 mg/kg), which is in the range commonly used for systemic delivery (1–3 mg/kg), but this dose did not show any ectopic mineralization. The broad data in this field are beginning to provide a compelling case for the utility of FK506 in numerous bone healing applications, both as a supplement to normal or engineered healing, and, with our findings, as a direct osteoinductive stimulus.
Although there are several small molecules that are capable of enhancing bone formation, and even enhancing BMP activity, few if any, possess the osteoinductive potency of BMP itself—that is, the ability to form de novo bone in a non-bone environment. This observation for FK506, a drug that has been cleared by the FDA for systemic use as an immunosuppressant, represents an attractive target to repurpose for a new paradigm of local bone healing. In future studies, it will be critical to test this potential in pre-clinical injury and degeneration models of bone repair (segmental defect repair and spine fusion). Furthering this knowledge has the potential to enable clinically successful bone healing to be achieved, thereby making this a safe technology that could be routinely used to improve bone healing in patients.
4. Materials and Methods
4.1. Cell Culture
Mouse C2C12 cells and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from ATCC (Manassas, VA, USA). The non-heat inactivated fetal bovine serum (FBS) was purchased from HyClone Laboratories, Inc. (Logan, UT, USA). The C2C12 cells at passages 4 to 7 were subcultured in T-75 cm2 flasks in DMEM supplemented with 10% FBS at 37 °C in 5% CO2 with humidification. When the flasks reached 60–70% confluence, the cells were trypsinized and seeded in triplicate at 200,000 cells/well in a 6-well plate for quantitative real-time RT-PCR and alkaline phosphatase (ALP) assays, or at 50,000 cells/well in a 12-well plate for the dual-luciferase reporter assay.
4.2. RNA Extraction and Reverse Transcription
The C2C12 cells were plated at a density of 200,000 cells/well in 6-well plates and grown overnight in DMEM containing 10% FBS. On day two, the culture medium was replaced with DMEM containing 2% FBS, and the cells were treated with various concentrations of the selected compound (diluted from 10 mg stock solutions prepared in DMSO) for 24 h. In control cultures, a DMSO solvent concentration of 0.01% (v/v) was applied. On day three, the medium was replaced with fresh DMEM containing 2% FBS, and the cells were treated with BMP-2 for 24 h. Total RNA was harvested using the RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen, Valencia, CA, USA). The harvested RNA was digested with RNase-free DNase I (Qiagen) to remove DNA contamination. The concentration of the isolated RNA was determined by measuring the absorbance at 260 nm wave length with a spectrophotometer (Model DU 640, Beckman Coulter, Inc. Brea, CA, USA). The ratio of A260/A280 was between 1.6 and 1.8. Reverse transcription was carried out to synthesize cDNA in a 100 μL volume with 2 μg of total RNA, 10× RT buffer, 5.5 mM MgCl2, 2 mM deoxynucleotide triphosphate (dNTP) mixture, 0.125 μM oligo d(T), 0.125 μM random primer, 40 units of RNase inhibitor, and 125 units of MultiScribe (Applied Biosystems, Foster City, CA, USA) for 10 min at 25 °C, 30 min at 48 °C, and 5 min at 95 °C.
4.3. Quantitative Real-Time RT-PCR
Quantitative real-time RT-PCR was performed to determine the mRNA expression level of early marker genes of BMP and TGFβ pathways. The sequences of the primers were as follows: Id1 (forward), 5′-GCGAGGTGGTACTTGGTCTG-3′; (reverse), 5′-GAGAGGGTGAGGCTCTGTTG-3′), TIEG1 (forward), 5′-GCCAACCATGCTCAACTTCG-3′; (reverse), 5′-TGCAGTTTTGTTCCAGGAATACAT-3′. Twenty-five microliters of reaction volume included 5 μL of cDNA, 0.5–10 μM of each primer, and 12.5 μL of 2× SYBR green master mix (Applied Biosystems). Real-time PCR was performed with the following three-step protocol: Step 1, 50 °C for 2 min; step 2, 95 °C for 10 min; step 3, and 40 cycles of 95 °C for 15 s and 62 °C for 1 min using the 7500 real-time PCR system (Applied Biosystems). To confirm the amplification specificity, the PCR products were subjected to a dissociation curve analysis. The threshold cycles (Ct) of each reaction were normalized to those obtained for 18S mRNA using the −ΔΔCt method (Applied Biosystems). All PCR reactions were performed in triplicate.
4.4. SDS-PAGE and Western Blotting
Cells were lysed to obtain total protein using Mammalian Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL, USA) or lysed to obtain nuclear protein using Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology) according to the manufacturer’s protocol. Each sample (10 μg of protein) was mixed with NuPage loading buffer (Invitrogen, Carlsbad, CA, USA) for a total volume of 20 μL and boiled for 5 min. The proteins were separated by electrophoresis under denaturing conditions on NuPage Bis-Tris Pre-Cast gels (Invitrogen,) for 60 min at 200 Volts and transferred onto nitrocellulose membranes (Invitrogen) for 60 min at 30 Volts. After the transfer, the membranes were incubated in 25 mL of blocking buffer (5% nonfat dry milk in Tris buffered saline (TBS)) for 1 h at room temperature. After blocking, membranes were washed three times for 5 min each in 15 mL of TBS with 0.1% Tween-20 (TBST). Washed membranes were incubated with different primary antibodies in TBST overnight at 4 °C. Anti-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Other antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). After incubation with primary antibody, membranes were washed three times for 5 min each with 10 mL of TBST. Washed membranes were incubated with HRP-conjugated anti-rabbit or anti-mouse secondary antibodies as indicated (1:2000, Cell Signaling Technology) in 10 mL of blocking buffer with gentle agitation for 1 h at room temperature. After incubation with secondary antibodies, membranes were washed three times for 5 min each with 10 mL of TBST. Washed membranes were incubated with 5 mL of SuperSignal West Pico Western blot substrate (Pierce Biotechnology) with gentle agitation for 4 min at room temperature. Membranes were drained of excess developing solution, wrapped in plastic wrap, and exposed to X-ray films.
4.5. Alkaline Phosphatase (ALP) Assay
The C2C12 cells were plated at 200,000 cells/well in 6-well plates and grown overnight in DMEM containing 10% FBS. On day two, the culture medium was replaced with DMEM containing 2% FBS and the cells were treated with 0.5 uM or an indicated concentration of compound for 24 h in 2 mL culture medium. On day three, the cells were treated with a final concentration of 50 ng/mL of BMP-2 with or without compound in DMEM medium containing 2% FBS for 72 h. The cells were washed with phosphate-buffered saline (PBS) and lysed by addition of lysis buffer (10 mM Tris-HCl pH 8.0, 1 mM MgCl2, and 0.5% Triton X-100). The cell lysates were centrifuged for 5 min at 13,000× g. The supernatant was collected and the aliquots were assayed for ALP activity and protein amount. The ALP activity was measured in triplicate using an ALP assay kit (Sigma-Aldrich) in microtiter plates. The protein amount was determined with Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA, USA) using bovine serum albumin (BSA) as a standard. The ALP activity (nmoles of p-nitrophenol per mL) was normalized to the protein amount (nmoles of p-nitrophenol per μg).
4.6. Calcineurin Activity Assay
Calcineurin activity was measured using a calcineurin cellular activity assay (Enzo Life Sciences, New York, NY, USA) according to manufacturer’s protocols. Mouse myoblastic C2C12 cell lysates were placed in a desalting column to remove excess phosphates and nucleotides. Total phosphatase activity was determined in the samples by incubating with a calcineurin-specific substrate (RII phosphopeptide, with sequence Asp-Leu-Asp-Val-Pro-Ile-Pro-Gly-Arg-Phe-Asp-Arg-Arg-Val-pSer-Val-Ala-Ala-Glu, designed from a subunit of the bovine cAMP-dependent protein kinase). A purified recombinant human calcineurin was used as a positive control. The detection of free-phosphate released was accomplished with Biomol Green reagent by monitoring absorbance at 620 nm in a SpectraMax M2 microplate reader (Molecular Devices, San Jose, CA, USA).
4.7. Ectopic Bone Formation Model
All animal procedures were approved by the local Institutional Animal Care and Use Committee. The FK506 compound was first tested in a standard rat chest ectopic bone formation model using a 5 μg/disc dose of rhBMP-2 as a positive control to induce bone formation consistently. rhBMP-2 or FK506 were loaded with the use of a pipette onto sterile bovine Type-I collagen disks (8 mm in diameter and 3 mm thick; Kensey Nash, Exton, PA, USA) in a biosafety cabinet. The disks were then transported in a sterile container to the surgical operating room. Each implant was loaded with a total volume of 100 uL solution containing 5 μg of rhBMP-2 (Medtronic, Minneapolis, MN, USA) or 100 μL of stock concentrations of 0, 10, 20, 30, and 40 mM of FK506 (corresponding to 0.8, 1.6, 2.4, and 3.2 mg of dry weight, respectively) solubilized in the organic solvent dimethyl sulfoxide (DMSO, Sigma-Aldrich) (n = 4 for each). In a pilot experiment, 10% to 100% DMSO was determined to have no effect on rhBMP-2-induced ectopic bone formation (data not shown).
Male Sprague Dawley five to six-week-old rats (Charles River Laboratories, Wilmington, MA, USA) were anesthetized with 3% to 5% isoflurane mixed with oxygen at a flow rate of 0.5 to 1 L/min and maintained during surgery with a dose of 1% to 2%. Surgery was performed with the animal positioned supine on a circulating-water heating pad. Two to four 1 cm transverse incisions were made about 3 cm apart on the chest of each rat, and subcutaneous pockets were created by blunt dissection with scissors. The implants were inserted into the pockets, and closure was accomplished with either 3-0 or 4-0 resorbable sutures (Vicryl; Ethicon, Johnson & Johnson, Somerville, NJ, USA) and/or skin clips. The rats were euthanized four weeks postoperatively. The implants were harvested and evaluated with manual palpation, high-resolution digital radiography, and non-decalcified histological analysis.
4.8. Digital Radiographic Evaluation
X-ray scanning (In-Vivo Xtreme, Bruker Corp., Billerica, MA, USA) was performed on the subcutaneous implants after fixation. The scans were executed with an exposure time of 1.2 s and a voltage of 45 kV. To assess bone formation, micro-computed tomography (microCT) scans (Micro-CT40, Scanco Medical, Bruttisellen, Switzerland) were performed. Samples were scanned with a 30 µM voxel size at a voltage of 45 kVp and a current of 177 μA. Three-dimensional reconstructions were obtained from evaluations of 500 slices of two-dimensional X-ray images.
4.9. Histological Analysis of Subcutaneous Ectopic Bone Formation
After euthanasia, at 4 weeks post-surgery, the subcutaneous implants were fixed with 10% formalin. Following fixation, the implants were washed and placed into a processor which dehydrated the samples sequentially in 70%, 95% and 100% alcohol, followed by xylene. The samples were then embedded in paraffin and cut into slices of 5 microns using a microtome (Accu-Cut SRM 200 Rotary Microtomoe, Sakura Finetek USA, Torrance, CA, USA). Slides were stained with Hematoxylin and Eosin (H&E) and Goldner’s trichrome (Sigma-Aldrich). Images were obtained with Lionheart LX (Biotek Instruments Inc., Winooski, VT, USA) at 4× and captured using Gen 5 software (Biotek Instruments Inc., Winooski, VT, USA)).
4.10. Statistics and Calculations
Results are presented as the mean of three determinations (n) with error bars representing the standard error of the mean (SEM). Experimental results that are visually represented are from consistent experiments where one representative experimental result is shown. Statistical significance (p < 0.05) was calculated using a one-way analysis of variance (ANOVA) with Bonferroni Post Hoc test (equal variances assumed) or Dunnett’s T3 Post Hoc test (equal variances not assumed) using Statistical Products for Social Sciences Version 16.0 (SPSS 16.0, IBM, Quarry Bay, HK, China) for Windows to compare various treatments in a multi-group analysis. A statistical probability of p < 0.05 was considered significant and is denoted as (a,b,c) in the figures.