Phosphoinositide 3 Kinase Signaling in Human Stem Cells from Reprogramming to Differentiation: A Tale in Cytoplasmic and Nuclear Compartments
Abstract
:1. Introduction
2. PI3K in Human Embryonic Stem Cell Pluripotency and iPSC Reprogramming
3. PI3K in Mesenchymal Stem Cell Differentiation
4. PI3K in Oral Mesenchymal Stem Cell Differentiation
5. Conclusions
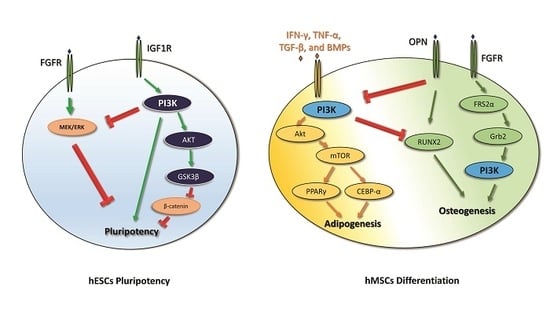
- iPSC reprogramming: PI3K seems to promote iPSC reprogramming by inhibiting GSK3β and FOXO1. Akt inhibition stops the reprogramming process of the cells, and, in the early steps of iPSC reprogramming, PI3K is involved in the switching from oxidative phosphorylation to glycolysis. The PI3K/Akt pathway also plays a pivotal role in the survival of iPSCs. The administration of Wortmannin, an inhibitor of PI3K/Akt signaling, induces apoptosis in iPSCs through caspase-3 activation.
- Adipogenic and osteogenic mesenchymal differentiation: During adipogenesis, the PI3K/Akt pathway is activated and its downstream mediators mTOR, FOXO1, p27, and p70S6K are increased. Indeed, the administration of the PI3K inhibitor LY294002 decreases the adipogenic differentiation of MSCs. Moreover, adipogenic differentiation requires a time-dependent modulation of the PI3K/Akt/mTOR pathway in order to promote autophagy-mediated differentiation. The PI3K pathway is involved in osteogenic differentiation as LY294002 partially suppresses this process, and it is associated with the MSC lineage commitment mediated by physical factors. Furthermore, the PI3k pathway promotes osteogenic differentiation of a particular type of MSC—ADSCs.
- Oral mesenchymal differentiation: In hDPSCs, the PI3K pathway is involved in the control of migration and it affects the expression of β-catenin and the phosphorylation of Akt and GSK3β. In addition, PI3K is involved in the response to physiological hypoxia in hDPSCs in vitro, where it controls intracellular ROS production and regulates oxidative stress. The PI3K/Akt pathway also controls the translocation of HIF-1α to the nucleus, since Akt inhibition causes inhibition in HIF-1α translocation. In PDLSCs, the PI3K/Akt pathway is involved in osteogenic differentiation promoted by different stimuli. For example, in osteogenic differentiation induced by mechanical stimuli, such as the activation of integrin α5/β1 by binding to a bioadhesive substrate, the isoform p110γ of PI3K interacts with integrin β1, and this interaction determines PI3K activation and the consequent induction of osteogenesis. Moreover, hPDLSCs can also differentiate into neural cells.
Funding
Conflicts of Interest
References
- Weissman, I.L. Stem cells: Units of development, units of regeneration, and units in evolution. Cell 2000, 100, 157–168. [Google Scholar] [CrossRef]
- Fu, X.; Xu, Y. Challenges to the clinical application of pluripotent stem cells: Towards genomic and functional stability. Genome Med. 2012, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.A.; Ross, E.G.; Bolli, R.; Pepine, C.J.; Leeper, N.J.; Yang, P.C. The Promise and Challenge of Induced Pluripotent Stem Cells for Cardiovascular Applications. JACC Basic Transl. Sci. 2016, 1, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Szade, K.; Szade, A.; Nowak, W.; Józkowicz, A. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim. Pol. 2015, 62, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.; Lee, S. Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 982. [Google Scholar] [CrossRef]
- Toker, A.; Cantley, L.C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 1997, 387, 673–676. [Google Scholar] [CrossRef]
- Bertacchini, J.; Frasson, C.; Chiarini, F.; D’Avella, D.; Accordi, B.; Anselmi, L.; Barozzi, P.; Foghieri, F.; Luppi, M.; Martelli, A.M.; et al. Dual inhibition of PI3K/mTOR signaling in chemoresistant AML primary cells. Adv. Biol. Regul. 2018, 68, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Hermida, M.A.; Dinesh Kumar, J.; Leslie, N.R. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv. Biol. Regul. 2017, 65, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Houdek, X.; Anderson, R.A. Phosphoinositide 3-kinase pathways and autophagy require phosphatidylinositol phosphate kinases. Adv. Biol. Regul. 2018, 68, 31–38. [Google Scholar] [CrossRef]
- Davis, W.J.; Lehmann, P.Z.; Li, W. Nuclear PI3K signaling in cell growth and tumorigenesis. Front. Cell Dev. Biol. 2015, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Dakhore, S.; Nayer, B.; Hasegawa, K. Human Pluripotent Stem Cell Culture: Current Status, Challenges, and Advancement. Stem Cells Int. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.B.; D’Amour, K.A.; Jones, K.L.; Krishnamoorthy, M.; Kulik, M.J.; Reynolds, D.M.; Sheppard, A.M.; Liu, H.; Xu, Y.; Baetge, E.E.; et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells 2007, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.M.; Reynolds, D.; Cliff, T.; Ohtsuka, S.; Mattheyses, A.L.; Sun, Y.; Menendez, L.; Kulik, M.; Dalton, S. Signaling network crosstalk in human pluripotent cells: A Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell 2012, 10, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.; Hughes, O.; Yung, S.; Hyslop, L.; Stewart, R.; Wappler, I.; Peters, H.; Walter, T.; Stojkovic, P.; Evans, J.; et al. The role of PI3K/AKT, MAPK/ERK and NFκβ signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum. Mol. Genet. 2006, 15, 1894–1913. [Google Scholar] [CrossRef]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef]
- Ding, V.M.Y.; Ling, L.; Natarajan, S.; Yap, M.G.S.; Cool, S.M.; Choo, A.B.H. FGF-2 modulates Wnt signaling in undifferentiated hESC and iPS cells through activated PI3-K/GSK3β signaling. J. Cell. Physiol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Furue, M.K.; Andrews, P.W. Inhibition of ERK1/2 prevents neural and mesendodermal differentiation and promotes human embryonic stem cell self-renewal. Stem Cell Res. 2010, 2, 157–169. [Google Scholar] [CrossRef]
- Hamilton, W.B.; Brickman, J.M. Erk Signaling Suppresses Embryonic Stem Cell Self-Renewal to Specify Endoderm. Cell Rep. 2014, 9, 2056–2070. [Google Scholar] [CrossRef]
- Oshimori, N.; Fuchs, E. The harmonies played by TGF-β in stem cell biology. Cell Stem Cell 2012, 11, 751–764. [Google Scholar] [CrossRef]
- Chen, Y.G.; Li, Z.; Wang, X.F. Where PI3K/Akt meets smads: The crosstalk determines human embryonic stem cell fate. Cell Stem Cell 2012, 10, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D. Signaling pathways dictating pluripotency in embryonic stem cells. Int. J. Dev. Biol. 2013, 57, 667–675. [Google Scholar] [CrossRef]
- Silva, J.; Barrandon, O.; Nichols, J.; Kawaguchi, J.; Theunissen, T.W.; Smith, A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008, 6), e253. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liang, D.; Tian, Q.; Chen, X.; Jiang, B.; Chou, B.K.; Hu, P.; Cheng, L.; Gao, P.; Li, J.; et al. Stimulation of somatic cell reprogramming by ERas-Akt-FoxO1 signaling axis. Stem Cells 2014, 32, 349–363. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, Z.; Luo, Y.; Zhao, X.; Wang, L.; Norris, C.; Tian, X.C. Differential effects of Akt isoforms on somatic cell reprogramming. J. Cell Sci. 2014, 127, 3998–4008. [Google Scholar] [CrossRef]
- Panopoulos, A.D.; Yanes, O.; Ruiz, S.; Kida, Y.S.; Diep, D.; Tautenhahn, R.; Herrerías, A.; Batchelder, E.M.; Plongthongkum, N.; Lutz, M.; et al. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 2012, 22, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Yeo, H.C.; Kang, N.Y.; Kim, H.; Lin, J.; Ha, H.H.; Vendrell, M.; Lee, J.S.; Chandran, Y.; Lee, D.Y.; et al. Mechanistic elements and critical factors of cellular reprogramming revealed by stepwise global gene expression analyses. Stem Cell Res. 2014, 12, 730–741. [Google Scholar] [CrossRef]
- Zhu, S.; Li, W.; Zhou, H.; Wei, W.; Ambasudhan, R.; Lin, T.; Kim, J.; Zhang, K.; Ding, S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 2010, 7, 651–655. [Google Scholar] [CrossRef]
- Wang, X.Q.; Lo, C.M.; Chen, L.; Ngan, E.S.W.; Xu, A.; Poon, R.Y.C. CDK1-PDK1-PI3K/Akt signaling pathway regulates embryonic and induced pluripotency. Cell Death Differ. 2017, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Rebelatto, C.K.; Aguiar, A.M.; Moretão, M.P.; Senegaglia, A.C.; Hansen, P.; Barchiki, F.; Oliveira, J.; Martins, J.; Kuligovski, C.; Mansur, F.; et al. Dissimilar Differentiation of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, and Adipose Tissue. Exp. Biol. Med. 2008, 233, 901–913. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Eswarakumar, V.P.; Lax, I.; Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005, 16, 139–149. [Google Scholar] [CrossRef]
- Jackson, R.A.; Nurcombe, V.; Cool, S.M. Coordinated fibroblast growth factor and heparan sulfate regulation of osteogenesis. Gene 2006, 379, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Yokota, J.; Chosa, N.; Sawada, S.; Okubo, N.; Takahashi, N.; Hasegawa, T.; Kondo, H.; Ishisaki, A. PDGF-induced PI3K-mediated signaling enhances the TGF-β-induced osteogenic differentiation of human mesenchymal stem cells in a TGF-β-activated MEK-dependent manner. Int. J. Mol. Med. 2014, 33, 534–542. [Google Scholar] [CrossRef]
- Kumar, A.; Salimath, B.P.; Stark, G.B.; Finkenzeller, G. Platelet-Derived Growth Factor Receptor Signaling Is Not Involved in Osteogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. A 2009, 16, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, T.; Fan, J.; Li, T.; Fan, L.; Wang, S.; Weng, X.; Han, Q.; Zhao, R.C. MIR-216a rescues dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT pathway. Cell Death Differ. 2015, 22, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Crawford, R.; Chen, C.; Xiao, Y. The Key Regulatory Roles of the PI3K/Akt Signaling Pathway in the Functionalities of Mesenchymal Stem Cells and Applications in Tissue Regeneration. Tissue Eng. Part B Rev. 2013, 19, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chen, Z.; Zhang, J.; Zhang, L.; Ke, H.; Huang, L.; Peng, Y.; Zhang, X.; Li, S.; Lahn, B.T.; et al. Critical role of phosphoinositide 3-kinase cascade in adipogenesis of human mesenchymal stem cells. Mol. Cell. Biochem. 2008, 310, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Jung, H.; Kim, D.; Kim, E.; Chung, J.; Cho, Y.; Park, S.; Park, B.; Ko, Y.; Bae, K.; et al. Regulation of adipogenic differentiation by LAR tyrosine phosphatase in human mesenchymal stem cells and 3T3-L1 preadipocytes. J. Cell Sci. 2009, 122, 4160–4167. [Google Scholar] [CrossRef]
- Song, B.Q.; Chi, Y.; Li, X.; Du, W.J.; Han, Z.B.; Tian, J.J.; Li, J.J.; Chen, F.; Wu, H.H.; Han, L.X.; et al. Inhibition of Notch Signaling Promotes the Adipogenic Differentiation of Mesenchymal Stem Cells Through Autophagy Activation and PTEN-PI3K/AKT/mTOR Pathway. Cell. Physiol. Biochem. 2015, 36, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Scioli, M.G.; Gentile, P.; Doldo, E.; Bonanno, E.; Spagnoli, L.G.; Orlandi, A. Platelet-Rich Plasma Greatly Potentiates Insulin-Induced Adipogenic Differentiation of Human Adipose-Derived Stem Cells Through a Serine/Threonine Kinase Akt-Dependent Mechanism and Promotes Clinical Fat Graft Maintenance. Stem Cells Transl. Med. 2012, 1, 206–220. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.-H.; Song, S.Y.; Kim, W.-S.; Song, S.U.; Yi, T.; Jeon, M.-S.; Chung, H.-M.; Xia, Y.; Sung, J.-H. Hypoxia induces adipocyte differentiation of adipose-derived stem cells by triggering reactive oxygen species generation. Cell Biol. Int. 2014, 38, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ruan, J.; Sun, Y.; Liu, M.; Sha, Z.; Fan, C.; Wu, Q. Long non-coding RNA HIF1A-AS2 facilitates adipose-derived stem cells (ASCs) osteogenic differentiation through miR-665/IL6 axis via PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2018, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Ramazzotti, G.; Fiume, R.; Chiarini, F.; Campana, G.; Ratti, S.; Billi, A.M.; Manzoli, L.; Follo, M.Y.; Suh, P.-G.G.; McCubrey, J.; et al. Phospholipase C-β1 interacts with cyclin E in adipose- derived stem cells osteogenic differentiation. Adv. Biol. Regul. 2019, 71, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ratti, S.; Mongiorgi, S.; Ramazzotti, G.; Follo, M.Y.; Mariani, G.A.; Suh, P.-G.; McCubrey, J.A.; Cocco, L.; Manzoli, L. Nuclear Inositide Signaling Via Phospholipase C. J. Cell. Biochem. 2017, 118, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Follo, M.Y.; Mongiorgi, S.; Finelli, C.; Clissa, C.; Ramazzotti, G.; Fiume, R.; Faenza, I.; Manzoli, L.; Martelli, A.M.; Cocco, L. Nuclear inositide signaling in myelodysplastic syndromes. J. Cell. Biochem. 2010, 109, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Manzoli, F.A.; Maraldi, N.M.; Cocco, L.; Capitani, S.; Facchini, A.; Facchinl, A. Chromatin phospholipids in normal and chronic lymphocytic leukemia lymphocytes. Cancer Res 1977, 37, 843–849. [Google Scholar]
- Manzoli, L.; Billi, A.M.; Gilmour, R.S.; Martelli, A.M.; Matteucci, A.; Rubbini, S.; Weber, G.; Cocco, L. Phosphoinositide signaling in nuclei of Friend cells: Tiazofurin down-regulates phospholipase C beta 1. Cancer Res 1995, 55, 2978–2980. [Google Scholar] [PubMed]
- Ratti, S.; Ramazzotti, G.; Faenza, I.; Fiume, R.; Mongiorgi, S.; Billi, A.M.A.M.; McCubrey, J.A.J.A.; Suh, P.-G.P.-G.; Manzoli, L.; Cocco, L.; et al. Nuclear inositide signaling and cell cycle. Adv. Biol. Regul. 2018, 67, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Fiume, R.; Baldanzi, G.; Capello, D.; Ratti, S.; Gesi, M.; Manzoli, L.; Graziani, A.; Suh, P.; Cocco, L.; et al. Nuclear Localization of Diacylglycerol Kinase Alpha in K562 Cells Is Involved in Cell Cycle Progression. J. Cell. Physiol. 2016, 232, 2550–2557. [Google Scholar] [CrossRef]
- De Jong, O.G.; van Balkom, B.W.M.; Schiffelers, R.M.; Bouten, C.V.C.; Verhaar, M.C. Extracellular vesicles: Potential roles in regenerative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef]
- Burger, D.; Viñas, J.L.; Akbari, S.; Dehak, H.; Knoll, W.; Gutsol, A.; Carter, A.; Touyz, R.M.; Allan, D.S.; Burns, K.D. Human Endothelial Colony-Forming Cells Protect against Acute Kidney Injury Role of Exosomes. Am. J. Pathol. 2015, 185, 2309–2323. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Chopp, M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell. Physiol. Biochem. 2018, 44, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.W.; Seo, M.K.; Woo, E.Y.; Kim, S.H.; Park, E.J.; Kim, S. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp. Dermatol. 2018, 27, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Otero-Ortega, L.; Gómez de Frutos, M.C.; Laso-García, F.; Rodríguez-Frutos, B.; Medina-Gutiérrez, E.; López, J.A.; Vázquez, J.; Díez-Tejedor, E.; Gutiérrez-Fernández, M. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2018, 5, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Li, H.; Zhou, Y.; Gu, B.; Xu, Y.; Fu, Q.; Peng, X.; Cao, N.; Fu, Q.; Jin, M.; et al. Therapeutic potential of human adipose-derived stem cell exosomes in stress urinary incontinence—An in vitro and in vivo study. Cell. Physiol. Biochem. 2018, 48, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Zhou, D.; Adams, L.L.; Wyss, B.K.; Shadday, M.D.; Woods, E.J.; Chu, T.M.G.; Goebel, W.S. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J. Tissue Eng. Regen. Med. 2010, 4, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Ma, L.; Jin, L.; Zhang, W.; Xiao, M.; Yu, Q. SDF-1/CXCR4 axis induces human dental pulp stem cell migration through FAK/PI3K/Akt and GSK3β/β-catenin pathways. Sci. Rep. 2017, 7, 40161. [Google Scholar] [CrossRef]
- Jiang, L.; Zhu, Y.Q.; Du, R.; Gu, Y.X.; Xia, L.; Qin, F.; Ritchie, H.H. The Expression and Role of Stromal Cell-derived Factor-1α-CXCR4 Axis in Human Dental Pulp. J. Endod. 2008, 34, 939–944. [Google Scholar] [CrossRef]
- Vieira, H.L.A.; Alves, P.M.; Vercelli, A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Prog. Neurobiol. 2011, 93, 444–455. [Google Scholar] [CrossRef]
- Lendahl, U.; Lee, K.L.; Yang, H.; Poellinger, L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat. Rev. Genet. 2009, 10, 821–832. [Google Scholar] [CrossRef]
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 2011, 8, 59–71. [Google Scholar] [CrossRef]
- Morimoto, H.; Iwata, K.; Ogonuki, N.; Inoue, K.; Atsuo, O.; Kanatsu-Shinohara, M.; Morimoto, T.; Yabe-Nishimura, C.; Shinohara, T. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell 2013, 12, 774–786. [Google Scholar] [CrossRef]
- Peng, L.; Wu, B.; Song, C.; Haider, F.; Luo, Z.; Liu, F.; Huang, X.; He, J.; Chen, T. Hypoxia-Activated PI3K/Akt Inhibits Oxidative Stress via the Regulation of Reactive Oxygen Species in Human Dental Pulp Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6595189. [Google Scholar]
- Kanichai, M.; Ferguson, D.; Prendergast, P.J.; Campbell, V.A. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: A role for AKT and hypoxia-inducible factor (HIF)-1α. J. Cell. Physiol. 2008, 216, 708–715. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Kapila, Y.; Lotz, J.; Kapila, S. Multiple Differentiation Capacity of STRO-1 + /CD146 + PDL Mesenchymal Progenitor Cells. Stem Cells Dev. 2009, 18, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Bright, R.; Hynes, K.; Gronthos, S.; Bartold, P.M. Periodontal ligament-derived cells for periodontal regeneration in animal models: A systematic review. J. Periodontal Res. 2015, 50, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Tsumanuma, Y.; Iwata, T.; Washio, K.; Yoshida, T.; Yamada, A.; Takagi, R.; Ohno, T.; Lin, K.; Yamato, M.; Ishikawa, I.; et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 2011, 32, 5819–5825. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Y.; Jia, L.; Ji, Y.; Zhao, B.; Wen, Y.; Xu, X. An In Vitro Comparative Study of Multisource Derived Human Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018, 27, 1634–1645. [Google Scholar] [CrossRef]
- Ge, B.; Liu, H.; Liang, Q.; Shang, L.; Wang, T.; Ge, S. Oxytocin facilitates the proliferation, migration and osteogenic differentiation of human periodontal stem cells in vitro. Arch. Oral Biol. 2019, 99, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; He, X.T.; Wang, J.; Li, X.; Xia, Y.; Tan, Y.Z.; Chen, F.M. Role of the P2X7 receptor in inflammation-mediated changes in the osteogenesis of periodontal ligament stem cells. Cell Death Dis. 2019, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Yi, J.K.; An, S.Y.; Heo, J.S. Increased osteogenic differentiation of periodontal ligament stem cells on polydopamine film occurs via activation of integrin and PI3K signaling pathways. Cell. Physiol. Biochem. 2014, 34, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Lanza Cariccio, V.; Scionti, D.; Raffa, A.; Iori, R.; Pollastro, F.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Treatment of periodontal ligament stem cells with MOR and CBD promotes cell survival and neuronal differentiation via the PI3K/Akt/mTOR pathway. Int. J. Mol. Sci. 2018, 19, 2341. [Google Scholar] [CrossRef] [PubMed]
- Trubiani, O.; Guarnieri, S.; Diomede, F.; Mariggiò, M.A.; Merciaro, I.; Morabito, C.; Cavalcanti, M.F.X.B.; Cocco, L.; Ramazzotti, G. Nuclear translocation of PKCα isoenzyme is involved in neurogenic commitment of human neural crest-derived periodontal ligament stem cells. Cell. Signal. 2016, 28, 1631–1641. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramazzotti, G.; Ratti, S.; Fiume, R.; Follo, M.Y.; Billi, A.M.; Rusciano, I.; Owusu Obeng, E.; Manzoli, L.; Cocco, L.; Faenza, I. Phosphoinositide 3 Kinase Signaling in Human Stem Cells from Reprogramming to Differentiation: A Tale in Cytoplasmic and Nuclear Compartments. Int. J. Mol. Sci. 2019, 20, 2026. https://doi.org/10.3390/ijms20082026
Ramazzotti G, Ratti S, Fiume R, Follo MY, Billi AM, Rusciano I, Owusu Obeng E, Manzoli L, Cocco L, Faenza I. Phosphoinositide 3 Kinase Signaling in Human Stem Cells from Reprogramming to Differentiation: A Tale in Cytoplasmic and Nuclear Compartments. International Journal of Molecular Sciences. 2019; 20(8):2026. https://doi.org/10.3390/ijms20082026
Chicago/Turabian StyleRamazzotti, Giulia, Stefano Ratti, Roberta Fiume, Matilde Yung Follo, Anna Maria Billi, Isabella Rusciano, Eric Owusu Obeng, Lucia Manzoli, Lucio Cocco, and Irene Faenza. 2019. "Phosphoinositide 3 Kinase Signaling in Human Stem Cells from Reprogramming to Differentiation: A Tale in Cytoplasmic and Nuclear Compartments" International Journal of Molecular Sciences 20, no. 8: 2026. https://doi.org/10.3390/ijms20082026
APA StyleRamazzotti, G., Ratti, S., Fiume, R., Follo, M. Y., Billi, A. M., Rusciano, I., Owusu Obeng, E., Manzoli, L., Cocco, L., & Faenza, I. (2019). Phosphoinositide 3 Kinase Signaling in Human Stem Cells from Reprogramming to Differentiation: A Tale in Cytoplasmic and Nuclear Compartments. International Journal of Molecular Sciences, 20(8), 2026. https://doi.org/10.3390/ijms20082026