Liquid Biopsy for the Diagnosis of Viral Hepatitis, Fatty Liver Steatosis, and Alcoholic Liver Diseases
Abstract
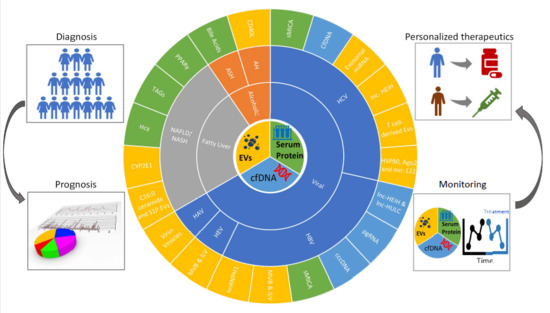
:1. Introduction
2. Liquid Biopsies for Viral Hepatitis
2.1. Liquid Biopsies Associated Viral Hepatitis Infections
2.2. Liquid Biopsies Associated Viral Hepatitis Immunity
2.3. Biomarkers of Liquid Biopsies Associated Viral Hepatitis
3. Liquid Biopsies for the Fatty Liver Disease
3.1. Biomarkers Associated Non-Alcoholic Steatohepatitis/Non-Alcoholic Fatty Liver Disease (NASH/NAFLD)
3.2. Hepatocyte-Derived Vesicles Associated Non-Alcoholic Steatohepatitis/Non-Alcoholic Fatty Liver Disease (NASH/NAFLD)
3.3. Liquid Biopsies Associated Chemoattraction in Non-Alcoholic Steatohepatitis/Non-Alcoholic Fatty Liver Disease (NASH/NAFLD)
4. Liquid Biopsies for Alcoholic Liver Disease (ALD)
4.1. Biomarkers Associated Alcoholic Liver Disease (ALD)
4.2. Hepatocyte-Derived Vesicles Associated Alcoholic Liver Disease (ALD)
4.3. Liquid Biopsies of Markers Associated with Inflammation in Alcoholic Liver Disease (ALD)
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine transaminase |
| AH | alcoholic hepatitis |
| ALD | alcoholic liver disease |
| ASH | alcoholic steatohepatitis |
| cfDNA | cell-free DNA |
| CCL2 | chemokine (C-C motif) ligand 2 |
| CXCL1 | chemokine (C-X-C motif) ligand 1 |
| CHB | chronic hepatitis B virus |
| CHC | chronic hepatitis C virus |
| cccDNA | covalently closed circular DNA |
| dsDNA | double-stranded DNA |
| ESCRT | endosomal sorting complexes required for transport |
| EV | extracellular vesicle |
| GWAS | genome-wide association study |
| HSC | hepatic stellate cell |
| HAV | hepatitis A virus |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| HGV | hepatitis G virus |
| HCC | hepatocellular carcinoma |
| Hcy | homocysteine |
| HuH | human hepatocyte-derived carcinoma cell |
| ILV | intraluminal vesicle |
| lncRNA | long non-coding RNA |
| LPC | lysophosphatidylcholine |
| LPC | lysophosphatidylcholine |
| MSC | mesenchymal stem cell |
| mRNA | messenger RNA |
| MICA | MHC class I chain-related A |
| MPs/MVs | microparticles/microvesicles |
| miRNA | microRNA |
| mtDNA | mitochondrial DNA |
| MVB | multivesicular body |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| PA | palmitic acid |
| PBMC | peripheral blood mononuclear cell |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| pgRNA | pre-genomic RNA |
| SNP | single nucleotide polymorphism |
| ssDNA | single-stranded DNA |
| sMICA | soluble MICA |
| S1P | sphingosine 1 phosphate |
| SK2 | sphingosine kinase 2 |
| TLR9 | toll-like receptor 9 |
| TAG | triacylglycerol |
| TG | triacylglycerol |
| TNFRSF10B | tumor necrosis factor receptor superfamily member 10B |
| VLDL | very-low-density lipoprotein |
| HSPG | heparan sulfate proteoglycans |
| L-SIGN | liver/lymph node-specific intracellular adhesion molecules-3 grabbing non-integrin |
| DC-SIGN | dendritic cell–specific ICAM-3 grabbing-nonintegrin |
| LDL-R | low-density lipoprotein receptor |
| TfR1 | transferrin receptor |
| NPC1L1 | NPC1-like intracellular cholesterol transporter 1 |
| CD81 | cluster of differentiation 81 |
| CLDN-1 | claudin 1 |
| SR-B1 | scavenger receptor class B type 1 |
| OCLN | occludin |
| Ago2 | argonaute RISC catalytic component 2 |
| ACC | acetyl-CoA carboxylase |
| ALKP | alkaline phosphatase |
| TNFα | tumor necrosis factor alpha |
| Tsg101 | tumor susceptibility 101 |
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Perumpail, B.J.; Khan, M.A.; Yoo, E.R.; Cholankeril, G.; Kim, D.; Ahmed, A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 8263–8276. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Hsu, C.T.; Yeh, M.L.; Huang, C.F.; Huang, C.I.; Liang, P.C.; Lin, Y.H.; Hsieh, M.Y.; Wei, Y.J.; Hsieh, M.H.; et al. Early fibrosis but late tumor stage and worse outcomes in hepatocellular carcinoma patients without hepatitis B or hepatitis C. Dig. Dis. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jun, T.W.; Yeh, M.L.; Yang, J.D.; Chen, V.L.; Nguyen, P.; Giama, N.H.; Huang, C.F.; Hsing, A.W.; Dai, C.Y.; Huang, J.F.; et al. More advanced disease and worse survival in cryptogenic compared to viral hepatocellular carcinoma. Liver Int. 2018, 38, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Lindenmeyer, C.C.; McCullough, A.J. The natural history of nonalcoholic fatty liver disease-an evolving view. Clin. Liver. Dis. 2018, 22, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.M.; Venkatesh, S.K.; Ehman, R.L.; Jhaveri, K.; Kamath, P.; Ohliger, M.A.; Samir, A.E.; Silva, A.C.; Taouli, B.; Torbenson, M.S.; et al. Evaluation of hepatic fibrosis: A review from the society of abdominal radiology disease focus panel. Abdom. Radiol. (NY) 2017, 42, 2037–2053. [Google Scholar] [CrossRef]
- Ratner, M. Jury out on liquid biopsies for cancer. Nat. Biotechnol. 2018, 36, 209–210. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: General methodologies and latest trends. Biomed. Res. Int. 2018, 8545347. [Google Scholar] [CrossRef]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell. Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [Green Version]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conde-Vancells, J.; Rodriguez-Suarez, E.; Embade, N.; Gil, D.; Matthiesen, R.; Valle, M.; Elortza, F.; Lu, S.C.; Mato, J.M.; Falcon-Perez, J.M. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J. Proteom. Res. 2008, 7, 5157–5166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, S.; Kim, J.; Jung, Y. Liver-derived exosomes and their implications in liver pathobiology. Int. J. Mol. Sci. 2018, 19, E3715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momen-Heravi, F.; Bala, S.; Kodys, K.; Szabo, G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci. Rep. 2015, 5, 9991. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Useros, N.; Puertas, M.C.; Borràs, F.E.; Blanco, J.; Martinez-Picado, J. Exosomes and retroviruses: The chicken or the egg? Cell Microbiol. 2011, 13, 10–17. [Google Scholar] [CrossRef]
- Grove, J.; Marsh, M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011, 195, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Moller-Tank, S.; Kondratowicz, A.S.; Davey, R.A.; Rennert, P.D.; Maury, W. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J. Virol. 2013, 87, 8327–8341. [Google Scholar] [CrossRef] [Green Version]
- Fitzner, D.; Schnaars, M.; van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.K.; Simons, M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011, 124, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Zhao, W.L.; Ye, Y.Y.; Bai, X.C.; Liu, R.Q.; Chang, L.F.; Zhou, Q.; Sui, S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic (Cph. Den.) 2010, 11, 675–687. [Google Scholar] [CrossRef]
- Miyanishi, M.; Tada, K.; Koike, M.; Uchiyama, Y.; Kitamura, T.; Nagata, S. Identification of Tim4 as a phosphatidylserine receptor. Nature 2007, 450, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Hensley, L.; McKnight, K.L.; Hu, F.; Madden, V.; Ping, L.; Jeong, S.H.; Walker, C.; Lanford, R.E.; Lemon, S.M. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013, 496, 367–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKnight, K.L.; Xie, L.; Gonzalez-Lopez, O.; Rivera-Serrano, E.E.; Chen, X.; Lemon, S.M. Protein composition of the hepatitis A virus quasi-envelope. Proc. Natl. Acad. Sci. USA 2017, 114, 6587–6592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagashima, S.; Jirintai, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Kouki, T.; Yashiro, T.; Okamoto, H. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J. Gen. Virol. 2014, 95, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. The ins and outs of hepatitis C virus entry and assembly. Nat. Rev. Microbiol. 2013, 11, 688–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukong, T.N.; Momen-Heravi, F.; Kodys, K.; Bala, S.; Szabo, G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014, 10, e1004424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meuleman, P.; Hesselgesser, J.; Paulson, M.; Vanwolleghem, T.; Desombere, I.; Reiser, H.; Leroux-Roels, G. Anti-CD81 antibodies can prevent a hepatitis C virus infection in vivo. Hepatology 2008, 48, 1761–1768. [Google Scholar] [CrossRef]
- Harman, A.N.; Nasr, N.; Feetham, A.; Galoyan, A.; Alshehri, A.A.; Rambukwelle, D.; Botting, R.A.; Hiener, B.M.; Diefenbach, E.; Diefenbach, R.J.; et al. HIV blocks interferon induction in human dendritic cells and macrophages by dysregulation of TBK1. J. Virol. 2015, 89, 6575–6584. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, N.; McLinden, J.H.; Xiang, J.; Landay, A.L.; Chivero, E.T.; Stapleton, J.T. GB virus C particles inhibit T cell activation via envelope E2 protein-mediated inhibition of TCR signaling. J. Immunol. 2013, 190, 6351–6359. [Google Scholar] [CrossRef] [Green Version]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Saha, B.; Kodys, K.; Szabo, G. Hepatitis C virus-induced monocyte differentiation into polarized M2 macrophages promotes stellate cell activation via TGF-beta. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 302–316. [Google Scholar] [CrossRef] [Green Version]
- Fusegawa, H.; Shiraishi, K.; Ogasawara, F.; Shimizu, M.; Haruki, Y.; Miyachi, H.; Matsuzaki, S.; Ando, Y. Platelet activation in patients with chronic hepatitis C. Tokai J. Exp. Clin. Med. 2002, 27, 101–106. [Google Scholar] [PubMed]
- Kornek, M.; Lynch, M.; Mehta, S.H.; Lai, M.; Exley, M.; Afdhal, N.H.; Schuppan, D. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology 2012, 143, 448–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Liu, K.; Liu, Y.; Xu, Y.; Zhang, F.; Yang, H.; Liu, J.; Pan, T.; Chen, J.; Wu, M.; et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat. Immunol. 2013, 14, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnaiah, V.; Thumann, C.; Fofana, I.; Habersetzer, F.; Pan, Q.; de Ruiter, P.E.; Willemsen, R.; Demmers, J.A.; Stalin Raj, V.; Jenster, G.; et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13109–13113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo, G.; Momen-Heravi, F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Dong, X.; Chen, Y.; Wang, X. Serum exosomal hnRNPH1 mRNA as a novel marker for hepatocellular carcinoma. Clin. Chem. Lab. Med. 2018, 56, 479–484. [Google Scholar] [CrossRef]
- Ruan, L.; Huang, L.; Zhao, L.; Wang, Q.; Pan, X.; Zhang, A.; Bai, Q.; Lv, Z. The Interaction of lncRNA-HEIH and lncRNA-HULC with HBXIP in hepatitis B patients. Gastroenterol. Res. Pract. 2018, 2018, 9187316. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, J.; Sun, M.; Yang, G.; Yuan, H.; Wang, Y.; Bu, Y.; Zhao, M.; Zhang, S.; Zhang, X. Long non-coding RNA HULC activates HBV by modulating HBx/STAT3/miR-539/APOBEC3B signaling in HBV-related hepatocellular carcinoma. Cancer Lett. 2019, 454, 158–170. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Qi, Q.; Gao, Y.; Wei, Q.; Han, S. lncRNA-HEIH in serum and exosomes as a potential biomarker in the HCV-related hepatocellular carcinoma. Cancer Biomark. 2018, 21, 651–659. [Google Scholar] [CrossRef]
- Lo, P.H.; Urabe, Y.; Kumar, V.; Tanikawa, C.; Koike, K.; Kato, N.; Miki, D.; Chayama, K.; Kubo, M.; Nakamura, Y.; et al. Identification of a functional variant in the MICA promoter which regulates MICA expression and increases HCV-related hepatocellular carcinoma risk. PLoS ONE 2013, 8, e61279. [Google Scholar] [CrossRef]
- Huang, C.F.; Wang, S.C.; Yeh, M.L.; Huang, C.I.; Tsai, P.C.; Lin, Z.Y.; Chen, S.C.; Dai, C.Y.; Huang, J.F.; Chuang, W.L.; et al. Association of serial serum major histocompatibility complex class I chain-related A measurements with hepatocellular carcinoma in chronic hepatitis C patients after viral eradication. J. Gastroenterol. Hepatol. 2019, 34, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Wang, S.C.; Chang, W.T.; Yeh, M.L.; Huang, C.I.; Lin, Z.Y.; Chen, S.C.; Chuang, W.L.; Huang, J.F.; Dai, C.Y.; et al. Lower protein expression levels of MHC class I chain-related gene A in hepatocellular carcinoma are at high risk of recurrence after surgical resection. Sci. Rep. 2018, 8, 15821. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yi Lo, P.H.; Sawai, H.; Kato, N.; Takahashi, A.; Deng, Z.; Urabe, Y.; Mbarek, H.; Tokunaga, K.; Tanaka, Y.; et al. Soluble MICA and a MICA variation as possible prognostic biomarkers for HBV-induced hepatocellular carcinoma. PLoS ONE 2012, 7, e44743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allweiss, L.; Dandri, M. The role of cccDNA in HBV maintenance. Viruses 2017, 9, E156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, N.; Ye, A.; Lin, J.; Liu, C.; Huang, J.; Fu, Y.; Wu, S.; Xu, S.; Wang, L.; Ou, Q. Diagnostic value of serum pgRNA detection in HBV-infected patients with different clinical outcomes. J. Clin. Microbiol. 2020, 58, e01275-19. [Google Scholar] [CrossRef] [PubMed]
- Tokuhisa, Y.; Iizuka, N.; Sakaida, I.; Moribe, T.; Fujita, N.; Miura, T.; Tamatsukuri, S.; Ishitsuka, H.; Uchida, K.; Terai, S.; et al. Circulating cell-free DNA as a predictive marker for distant metastasis of hepatitis C virus-related hepatocellular carcinoma. Br. J. Cancer 2007, 97, 1399–1403. [Google Scholar] [CrossRef]
- Iida, M.; Iizuka, N.; Sakaida, I.; Moribe, T.; Fujita, N.; Miura, T.; Tamatsukuri, S.; Ishitsuka, H.; Uchida, K.; Terai, S.; et al. Relation between serum levels of cell-free DNA and inflammation status in hepatitis C virus-related hepatocellular carcinoma. Oncol. Rep. 2008, 20, 761–765. [Google Scholar]
- Ng, C.K.Y.; Di Costanzo, G.G.; Terracciano, L.M.; Piscuoglio, S. Circulating cell-free DNA in hepatocellular carcinoma: Current insights and outlook. Front. Med. (Lausanne) 2018, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Brumbaugh, D.E.; Friedman, J.E. Developmental origins of nonalcoholic fatty liver disease. Pediatr. Res. 2014, 75, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic fatty liver disease: Basic pathogenetic mechanisms in the progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Chan, S.L.; Wong, A.M.; Lee, K.; Wong, N.; Chan, A.K. Personalized therapy for hepatocellular carcinoma: Where are we now? Cancer Treat. Rev. 2016, 45, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.X.; Hu, C.X.; Sun, W.L.; Pan, Q.; Shen, F.; Yang, Z.; Su, Q.; Xu, G.W.; Fan, J.G. Serum monounsaturated triacylglycerol predicts steatohepatitis in patients with non-alcoholic fatty liver disease and chronic hepatitis B. Sci. Rep. 2017, 7, 10517. [Google Scholar] [CrossRef] [Green Version]
- Fartoux, L.; Chazouillères, O.; Wendum, D.; Poupon, R.; Serfaty, L. Impact of steatosis on progression of fibrosis in patients with mild hepatitis C. Hepatology 2005, 41, 82–87. [Google Scholar] [CrossRef]
- Mehta, S.R.; Thomas, E.L.; Bell, J.D.; Johnston, D.G.; Taylor-Robinson, S.D. Non-invasive means of measuring hepatic fat content. World J. Gastroenterol. 2008, 14, 3476–3483. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.; Zeybel, M.; Day, C.P.; Dipper, C.; Masson, S.; McPherson, S.; Henderson, E.; Tiniakos, D.; White, S.; French, J.; et al. Plasma DNA methylation: A potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut 2017, 66, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, S.C.; Muniz, M.T.; Siqueira, M.D.; Siqueira, E.R.; Gomes, A.V.; Silva, K.A.; Bezerra, L.C.; D’Almeida, V.; de Oliveira, C.P.; Pereira, L.M. Plasmatic higher levels of homocysteine in non-alcoholic fatty liver disease (NAFLD). Nutr. J. 2013, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, M.; Yesilova, Z.; Bagci, S.; Uygun, A.; Ozcan, A.; Ercin, C.N.; Erdil, A.; Sanisoglu, S.Y.; Cakir, E.; Ates, Y.; et al. Elevated plasma homocysteine concentrations as a predictor of steatohepatitis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2005, 20, 1448–1455. [Google Scholar] [CrossRef]
- Chang, B.; Xu, M.J.; Zhou, Z.; Cai, Y.; Li, M.; Wang, W.; Feng, D.; Bertola, A.; Wang, H.; Kunos, G.; et al. Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: An important role for CXCL1. Hepatology 2015, 62, 1070–1085. [Google Scholar] [CrossRef] [Green Version]
- Chashmniam, S.; Ghafourpour, M.; Rezaei Farimani, A.; Gholami, A.; Nobakht Motlagh Ghoochani, B.F. Metabolomic Biomarkers in the Diagnosis of Non-Alcoholic Fatty Liver Disease. Hepat. Mon. 2019, 19, e92244. [Google Scholar] [CrossRef] [Green Version]
- Morgan, K.; Uyuni, A.; Nandgiri, G.; Mao, L.; Castaneda, L.; Kathirvel, E.; French, S.W.; Morgan, T.R. Altered expression of transcription factors and genes regulating lipogenesis in liver and adipose tissue of mice with high fat diet-induced obesity and nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2008, 20, 843–854. [Google Scholar] [CrossRef]
- Yoshida, M. Novel role of NPC1L1 in the regulation of hepatic metabolism: Potential contribution of ezetimibe in NAFLD/NASH treatment. Curr. Vasc. Pharmacol. 2011, 9, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, M.F.; Jiang, S.; Wu, J.; Liu, J.; Yuan, X.W.; Shen, D.; Zhang, J.Z.; Zhou, N.; He, J.; et al. Liver governs adipose remodeling via extracellular vesicles in response to lipid overload. Nat. Commun. 2020, 11, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.H.; Hirsova, P.; Tomita, K.; Bronk, S.F.; Werneburg, N.W.; Harrison, S.A.; Goodfellow, V.S.; Malhi, H.; Gores, G.J. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology 2016, 63, 731–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povero, D.; Eguchi, A.; Li, H.; Johnson, C.D.; Papouchado, B.G.; Wree, A.; Messer, K.; Feldstein, A.E. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS ONE 2014, 9, e113651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsova, P.; Ibrahim, S.H.; Krishnan, A.; Verma, V.K.; Bronk, S.F.; Werneburg, N.W.; Charlton, M.R.; Shah, V.H.; Malhi, H.; Gores, G.J. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology 2016, 150, 956–967. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Martinez, I.; Santoro, N.; Chen, Y.; Hoque, R.; Ouyang, X.; Caprio, S.; Shlomchik, M.J.; Coffman, R.L.; Candia, A.; Mehal, W.Z. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J. Clin. Investig. 2016, 126, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Tosello-Trampont, A.C.; Landes, S.G.; Nguyen, V.; Novobrantseva, T.I.; Hahn, Y.S. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J. Biol. Chem. 2012, 287, 40161–40172. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.H.; Gores, G.J.; Hirsova, P.; Kirby, M.; Miles, L.; Jaeschke, A.; Kohli, R. Mixed lineage kinase 3 deficient mice are protected against the high fat high carbohydrate diet-induced steatohepatitis. Liver Int. 2014, 34, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Mridha, A.R.; Haczeyni, F.; Yeh, M.M.; Haigh, W.G.; Ioannou, G.N.; Barn, V.; Ajamieh, H.; Adams, L.; Hamdorf, J.M.; Teoh, N.C.; et al. TLR9 is up-regulated in human and murine NASH: Pivotal role in inflammatory recruitment and cell survival. Clin. Sci. (Lond.) 2017, 131, 2145–2159. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, S.Y.; Ko, E.; Lee, J.H.; Yi, H.S.; Yoo, Y.J.; Je, J.; Suh, S.J.; Jung, Y.K.; Kim, J.H.; et al. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci. Rep. 2017, 7, 3710. [Google Scholar] [CrossRef]
- Witek, R.P.; Yang, L.; Liu, R.; Jung, Y.; Omenetti, A.; Syn, W.K.; Choi, S.S.; Cheong, Y.; Fearing, C.M.; Agboola, K.M.; et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology 2009, 136, 320–330 e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.B.; Liu, Y.; Liu, C.; Xiang, X.; Wang, J.; Cheng, Z.; Shah, S.V.; Zhang, S.; Zhang, L.; Zhuang, X.; et al. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology 2009, 50, 1412–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 2019, 8, E1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, A.; Mulya, A.; Lazic, M.; Radhakrishnan, D.; Berk, M.P.; Povero, D.; Gornicka, A.; Feldstein, A.E. Microparticles release by adipocytes act as “find-me” signals to promote macrophage migration. PLoS ONE 2015, 10, e0123110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakazu, E.; Mauer, A.S.; Yin, M.; Malhi, H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J. Lipid. Res. 2016, 57, 233–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaggini, M.; Carli, F.; Rosso, C.; Younes, R.; D’Aurizio, R.; Bugianesi, E.; Gastaldelli, A. Altered Metabolic Profile and Adipocyte Insulin Resistance Mark Severe Liver Fibrosis in Patients with Chronic Liver Disease. Int. J. Mol. Sci. 2019, 20, 6333. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Tsukamoto, H. Inflammation in alcoholic and nonalcoholic fatty liver disease: Friend or foe? Gastroenterology 2016, 150, 1704–1709. [Google Scholar] [CrossRef] [Green Version]
- Mills, S.J.; Harrison, S.A. Comparison of the natural history of alcoholic and nonalcoholic fatty liver disease. Curr. Gastroenterol. Rep. 2005, 7, 32–36. [Google Scholar] [CrossRef]
- Trinchet, J.C.; Gerhardt, M.F.; Balkau, B.; Munz, C.; Poupon, R.E. Serum bile acids and cholestasis in alcoholic hepatitis. Relationship with usual liver tests and histological features. J. Hepatol. 1994, 21, 235–240. [Google Scholar] [CrossRef]
- Moratti, E.; Vezzalini, M.; Tomasello, L.; Giavarina, D.; Sorio, C. Identification of protein tyrosine phosphatase receptor gamma extracellular domain (sPTPRG) as a natural soluble protein in plasma. PLoS ONE 2015, 10, e0119110. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.J.; Cai, Y.; Wang, H.; Altamirano, J.; Chang, B.; Bertola, A.; Odena, G.; Lu, J.; Tanaka, N.; Matsusue, K.; et al. Fat-specific protein 27/CIDEC promotes development of alcoholic steatohepatitis in mice and humans. Gastroenterology 2015, 149, 1030–1041.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momen-Heravi, F.; Saha, B.; Kodys, K.; Catalano, D.; Satishchandran, A.; Szabo, G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J. Tranl. Med. 2015, 13, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.E.; Mezey, E.; Hardwick, J.P.; Salem, N., Jr.; Clemens, D.L.; Song, B.J. Increased ethanol-inducible cytochrome P450-2E1 and cytochrome P450 isoforms in exosomes of alcohol-exposed rodents and patients with alcoholism through oxidative and endoplasmic reticulum stress. Hepatol. Commun. 2017, 1, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Kugler, V.; Bode, J.C. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatol. 1987, 4, 8–14. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, M.J.; Koritzinsky, E.H.; Zhou, Z.; Wang, W.; Cao, H.; Yuen, P.S.; Ross, R.A.; Star, R.A.; Liangpunsakul, S.; et al. Mitochondrial DNA-enriched microparticles promote acute-on-chronic alcoholic neutrophilia and hepatotoxicity. JCI Insight 2017, 2, 92634. [Google Scholar] [CrossRef]
- Saha, B.; Momen-Heravi, F.; Furi, I.; Kodys, K.; Catalano, D.; Gangopadhyay, A.; Haraszti, R.; Satishchandran, A.; Iracheta-Vellve, A.; Adejumo, A.; et al. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation through heat shock protein 90. Hepatology 2018, 67, 1986–2000. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Suárez, E.; Gonzalez, E.; Hughes, C.; Conde-Vancells, J.; Rudella, A.; Royo, F.; Palomo, L.; Elortza, F.; Lu, S.C.; Mato, J.M.; et al. Quantitative proteomic analysis of hepatocyte-secreted extracellular vesicles reveals candidate markers for liver toxicity. J. Proteom. 2014, 103, 227–240. [Google Scholar] [CrossRef]
- Cho, Y.E.; Im, E.J.; Moon, P.G.; Mezey, E.; Song, B.J.; Baek, M.C. Increased liver-specific proteins in circulating extracellular vesicles as potential biomarkers for drug- and alcohol-induced liver injury. PLoS ONE 2017, 12, e0172463. [Google Scholar] [CrossRef]
- Bissonnette, J.; Altamirano, J.; Devue, C.; Roux, O.; Payancé, A.; Lebrec, D.; Bedossa, P.; Valla, D.; Durand, F.; Ait-Oufella, H.; et al. A prospective study of the utility of plasma biomarkers to diagnose alcoholic hepatitis. Hepatology 2017, 66, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Sukriti, S.; Maras, J.S.; Bihari, C.; Das, S.; Vyas, A.K.; Sharma, S.; Hussain, S.; Shasthry, S.; Choudhary, A.; Premkumar, M.; et al. Microvesicles in hepatic and peripheral vein can predict nonresponse to corticosteroid therapy in severe alcoholic hepatitis. Aliment Pharmacol. Ther. 2018, 47, 1151–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.K.; Li, H.; Wang, R.; Hirsova, P.; Mushref, M.; Liu, Y.; Cao, S.; Contreras, P.C.; Malhi, H.; Kamath, P.S.; et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J. Hepatol. 2016, 64, 651–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, Y. The role of chemokines in neutrophil biology. Front. Biosci. 2008, 13, 2400–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista, A.P. Neutrophilic infiltration in alcoholic hepatitis. Alcohol 2002, 27, 17–21. [Google Scholar] [CrossRef]
- Dominguez, M.; Miquel, R.; Colmenero, J.; Moreno, M.; García-Pagán, J.C.; Bosch, J.; Arroyo, V.; Ginès, P.; Caballería, J.; Bataller, R. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology 2009, 136, 1639–1650. [Google Scholar] [CrossRef]
- Sheron, N.; Bird, G.; Koskinas, J.; Portmann, B.; Ceska, M.; Lindley, I.; Williams, R. Circulating and tissue levels of the neutrophil chemotaxin interleukin-8 are elevated in severe acute alcoholic hepatitis, and tissue levels correlate with neutrophil infiltration. Hepatology 1993, 18, 41–46. [Google Scholar]
- Roh, Y.S.; Zhang, B.; Loomba, R.; Seki, E. TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G30–G41. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, T.N.; Leung, C.A.; Douti, L.Y.; Jay, S.M. Ethanol induces enhanced vascularization bioactivity of endothelial cell-derived extracellular vesicles via regulation of microRNAs and long non-coding RNAs. Sci. Rep. 2017, 7, 13794. [Google Scholar] [CrossRef]




| References | Biomarker | Type of Liquid Biopsy | Application | Disease | Source | Sample Type |
|---|---|---|---|---|---|---|
| McKnight, Xie et al. [22] | MVB | Exosomes | Viral infection | HAV | Infected cells | Serum |
| J Gen Virol 2014, Nagashima S. et al. [23] | MVB | Exosomes | Viral infection | HEV | Infected cells | Cell supernatants |
| PLoS Pathog 2014, Bukong TN. et al. [25] | HSP90, Ago2, and mir-122 | Exosomes | Viral infection | HCV | Infected cells | Serum/Cell supernatants |
| Clin Chem Lab Med. 2018, Xu H. et al. [36] | Exosome-hnRNPH1 | Exosomes | Biomarker | HBV | Hepatocyte | Serum |
| Gastroenterol. Res. Pract. 2018, Ruan, L. et al. [37] | lncRNA-HEIH & lncRNA-HULC | - | Biomarker | HBV | Hepatocyte | Peripheral blood |
| Cancer Biomark 2018, Zhang C. et al. [39] | lncRNA-HEIH | Exosomal | Biomarker | HCV | Hepatocyte | Serum |
| J Gastroenterol Hepatol 2019, Huang CF. et al. [41] | sMICA | Protein | Biomarker | HCV | Infected cells | Serum |
| PLoS One. 2012, Kumar V. et al. [43] | sMICA | protein | Biomarker | HBV | Hepatocyte | Serum |
| Viruses. 2017, Allweiss L. et al. [44] | cccDNA | Viral DNA | Diagnosis | HBV | Virus | Serum/Cell supernatants |
| Journal of Clinical Microbiology 2019, Lin N. et al. [45] | pgRNA | Viral RNA | Diagnosis | HBV | Virus | Serum |
| Br J Cancer 2007, Tokuhisa Y. et al. [46] | cfDNA | HCC DNA | Biomarker | HCV | Hepatocyte | Serum/plasma |
| Gastroenterology 2012, Kornek M. et al. [32] | Levels | Microparticles | Hepatic inflammation | HCV | - | Serum/plasma |
| References [ref] | Biomarker | Type of Liquid Biopsy | Application | Disease | Source | Sample Type |
|---|---|---|---|---|---|---|
| Hepatology. 2016, Ibrahim, S.H. et al. [63] | Toxic-Lipids and CYP2E1 | EVs | Prognostic Biomarker | NASH | Hepatocyte | Cell Supernatants/Mouse Model Serum |
| Clin Sci (Lond) 2017, Mridha, Haczeyni et al. [69] | TLR9 | EVs | Diagnostic & prognostic biomarker | NASH | Hepatocytes | Cell supernatants |
| J Lipid Res 2016, Kakazu E. et al. [75] | C16:0 ceramide and S1P | EVs | Diagnosis & monitoring biomarker | NASH | Hepatocytes | Mouse model Serum |
| Gastroenterology 2012, Kornek M. et al. [32] | Levels | Microparticles | Hepatic inflammation | NAFLD | - | Serum/plasma |
| J Clin Invest 2016, Garcia-Martinez, Santoro et al. [66] | mtDNA | Microparticles | Diagnostic & prognostic biomarkers | NASH | Hepatocytes | Plasma |
| Sci Rep. 2017, Yang RX. et al. [52] | Triacylglycerol | Protein | Diagnosis biomarker | NAFLD | - | Serum |
| J Gastroenterol Hepatol. 2005, Gulsen, M. et al. [57] | Hcy | Protein | Predictor biomarkers | NAFLD | Hepatocytes | plasma |
| Gut. 2017, Hardy T. et al. [55] | PPARγ | DNA methylation | Prognostic biomarker | NAFLD | - | Plasma |
| References [ref] | Biomarker | Type of Liquid Biopsy | Application | Disease | Source | Sample Type |
|---|---|---|---|---|---|---|
| Hepatol 1994, [ref] Trinchet JC. et al. [80] | Bile acids | Protein | Diagnosis biomarker | ASH | - | Serum |
| PLoS One 2015, Moratti, Vezzalini et al. [81] | sPTPRG | Protein | Prognosis biomarker | AH | - | Plasma |
| J Transl Med 2015, Momen-Heravi, Saha et al. [83] | miR-192 and miR-30a | Exosomes | Predictor biomarker | AH | - | Serum |
| JCI Insight 2017, Cai, Xu et al. [86] | mtDNA | Microparticles | Prognosis biomarker | AH | - | Serum |
| Hepatology 2018, Saha, Momen-Heravi et al. [87] | CCL2 | EVs | Prognosis biomarker | AH | - | Mouse model Serum |
| PLoS One 2017, Cho, Im et al. [89] | Liver-specific proteins | EVs | Prognosis biomarker | AH | Hepatocytes | plasma |
| Aliment Pharmacol Ther 2018, Sukriti, Maras et al. [91] | CD34+ and ASGPR+ | MVs | Prognosis biomarker | AH | haematopoietic stem-cells, hepatocytes. | Serum |
| Journal of Hepatology 2016, Verma VK. et al. [92] | CD40L | EVs | Prognosis biomarker | AH | Hepatocytes | Serum |
| Sci Rep 2017, Lamichhane, Leung et al. [98] | lncRNA-HOTAIR lncRNA-MALAT1 | EVs | Prognosis biomarker | AH | Endothelial cells | Mouse model Serum |
| Hepatology 2015, Chang, Xu et al. [58] | CXCL1 | - | Prognosis biomarker | ALD | - | Serum |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabangu, C.S.; Huang, J.-F.; Hsiao, H.-H.; Yu, M.-L.; Chuang, W.-L.; Wang, S.-C. Liquid Biopsy for the Diagnosis of Viral Hepatitis, Fatty Liver Steatosis, and Alcoholic Liver Diseases. Int. J. Mol. Sci. 2020, 21, 3732. https://doi.org/10.3390/ijms21103732
Shabangu CS, Huang J-F, Hsiao H-H, Yu M-L, Chuang W-L, Wang S-C. Liquid Biopsy for the Diagnosis of Viral Hepatitis, Fatty Liver Steatosis, and Alcoholic Liver Diseases. International Journal of Molecular Sciences. 2020; 21(10):3732. https://doi.org/10.3390/ijms21103732
Chicago/Turabian StyleShabangu, Ciniso Sylvester, Jee-Fu Huang, Hui-Hua Hsiao, Ming-Lung Yu, Wan-Long Chuang, and Shu-Chi Wang. 2020. "Liquid Biopsy for the Diagnosis of Viral Hepatitis, Fatty Liver Steatosis, and Alcoholic Liver Diseases" International Journal of Molecular Sciences 21, no. 10: 3732. https://doi.org/10.3390/ijms21103732
APA StyleShabangu, C. S., Huang, J. -F., Hsiao, H. -H., Yu, M. -L., Chuang, W. -L., & Wang, S. -C. (2020). Liquid Biopsy for the Diagnosis of Viral Hepatitis, Fatty Liver Steatosis, and Alcoholic Liver Diseases. International Journal of Molecular Sciences, 21(10), 3732. https://doi.org/10.3390/ijms21103732