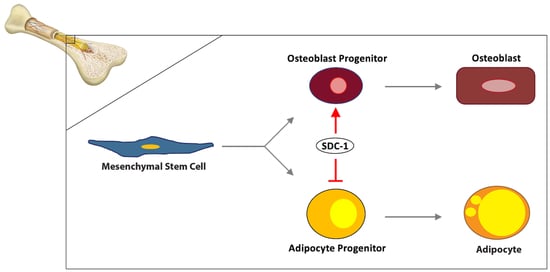
Syndecan-1 Facilitates the Human Mesenchymal Stem Cell Osteo-Adipogenic Balance
Abstract
:1. Introduction
2. Results
2.1. Disrupted HSPG Profile Impedes Undifferentiated hMSC Proliferation
2.2. Expression of HS Biosynthetic Machinery and HSPG Core Protein in Niche-Altered hMSC Cultures
2.3. SDC-1 KD Led to the Downregulation of Osteogenic Markers and up-Regulation of Adipogenic Markers
2.4. BMP2 Signalling Was Induced Temporally Following SDC-1 KD in Undifferentiated hMSC
2.5. Enhanced Adipogenesis and Impaired Osteoblast Maturation Was Observed in hMSC SDC-1 KDAD/OS Cultures
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Niche Modification
4.2. Adipogenic Differentiation and SDC-1 Knockdown
4.3. Oil Red O Staining
4.4. Osteogenic Differentiation and SDC-1 Knockdown
4.5. Alizarin Red and von Kossa Staining
4.6. Quantitation of hMSC Lineage Differentiated Stained Cultures
4.7. RNA Isolation and cDNA Synthesis
4.8. Quantitative Real-Time PCR
4.9. Western Blotting
4.10. Ethical Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pittenger, M.F. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minguell, J.J.; Erices, A.; Conget, P. Mesenchymal Stem Cells. Exp. Boil. Med. 2001, 226, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, M.; Klibanski, A. Anorexia nervosa, obesity and bone metabolism. Pediatr. Endocrinol. Rev. 2013, 11, 21–33. [Google Scholar]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Motyl, K.J.; Raetz, M.; Tekalur, S.A.; Schwartz, R.C.; McCabe, L.R. CCAAT/enhancer binding protein β-deficiency enhances type 1 diabetic bone phenotype by increasing marrow adiposity and bone resorption. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R1250–R1260. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, M. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr. Opin. Pharmacol. 2004, 4, 290–294. [Google Scholar] [CrossRef]
- Georgiou, K.; Scherer, M.; Fan, C.-M.; Cool, J.C.; King, T.; Foster, B.K.; Xian, C.J. Methotrexate chemotherapy reduces osteogenesis but increases adipogenic potential in the bone marrow. J. Cell. Physiol. 2011, 227, 909–918. [Google Scholar] [CrossRef]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Perrien, D.; Akel, N.S.; Dupont-Versteegden, E.E.; Skinner, R.A.; Siegel, E.R.; Suva, L.J.; Gaddy, D. Aging alters the skeletal response to disuse in the rat. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R988–R996. [Google Scholar] [CrossRef] [Green Version]
- Di Iorgi, N.; Rosol, M.; Mittelman, S.D.; Gilsanz, V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J. Clin. Endocrinol. Metab. 2008, 93, 2281–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, S.K.; Moore, S.G.; Kaplan, I.D. Early and late bone-marrow changes after irradiation: MR evaluation. Am. J. Roentgenol. 1990, 154, 745–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wren, T.; Chung, S.A.; Adams, G.B.; Gilsanz, V.; Dorey, F.J.; Bluml, S. Bone Marrow Fat Is Inversely Related to Cortical Bone in Young and Old Subjects. J. Clin. Endocrinol. Metab. 2011, 96, 782–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozario, T.; DeSimone, U.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Boil. 2010, 341, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, A.D.; Olsen, B.R. Osteoblast-adipocyte lineage plasticity in tissue development, maintenance and pathology. Cell. Mol. Life Sci. 2013, 71, 493–497. [Google Scholar] [CrossRef] [Green Version]
- MacDougald, O.; Mandrup, S. Adipogenesis: Forces that tip the scales. Trends Endocrinol. Metab. 2002, 13, 5–11. [Google Scholar] [CrossRef]
- Rosen, E.; MacDougald, O. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Boil. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Chen, X. Extracellular matrix provides an optimal niche for the maintenance and propagation of mesenchymal stem cells. Birth Defects Res. Part C Embryo Today Rev. 2010, 90, 45–54. [Google Scholar] [CrossRef]
- Kirn-Safran, C.; Farach-Carson, M.C.; Carson, D.D. Multifunctionality of extracellular and cell surface heparan sulfate proteoglycans. Cell. Mol. Life Sci. 2009, 66, 3421–3434. [Google Scholar] [CrossRef]
- Lin, X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development 2004, 131, 6009–6021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Boil. 2011, 3, a004952. [Google Scholar] [CrossRef] [Green Version]
- Okolicsanyi, R.K.; Van Wijnen, A.J.; Cool, S.M.; Stein, J.L.; Griffiths, L.R.; Haupt, L.M. Heparan sulfate proteoglycans and human breast cancer epithelial cell tumorigenicity. J. Cell. Biochem. 2014, 115, 967–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cool, S.M.; Nurcombe, V. Heparan sulfate regulation of progenitor cell fate. J. Cell. Biochem. 2006, 99, 1040–1051. [Google Scholar] [CrossRef]
- Haupt, L.M.; Murali, S.; Mun, F.K.; Teplyuk, N.; Mei, L.F.; Stein, G.S.; Van Wijnen, A.J.; Nurcombe, V.; Cool, S.M. The heparan sulfate proteoglycan (HSPG) glypican-3 mediates commitment of MC3T3-E1 cells toward osteogenesis. J. Cell. Physiol. 2009, 220, 780–791. [Google Scholar] [CrossRef]
- Jackson, R.A.; Nurcombe, V.; Cool, S.M. Coordinated fibroblast growth factor and heparan sulfate regulation of osteogenesis. Gene 2006, 379, 79–91. [Google Scholar] [CrossRef]
- Mansouri, R.; Haÿ, E.; Marie, P.J.; Modrowski, D. Role of syndecan-2 in osteoblast biology and pathology. BoneKEy Rep. 2015, 4, 666. [Google Scholar] [CrossRef] [Green Version]
- Molténi, A.; Modrowski, D.; Hott, M.; Marie, P.J. Alterations of matrix- and cell-associated proteoglycans inhibit osteogenesis and growth response to fibroblast growth factor-2 in cultured rat mandibular condyle and calvaria. Cell Tissue Res. 1999, 295, 523–536. [Google Scholar] [CrossRef]
- Teplyuk, N.M.; Haupt, L.M.; Ling, L.; Dombrowski, C.; Mun, F.K.; Nathan, S.S.; Lian, J.B.; Stein, J.L.; Stein, G.S.; Cool, S.M.; et al. The osteogenic transcription factor Runx2 regulates components of the fibroblast growth factor/proteoglycan signaling axis in osteoblasts. J. Cell. Biochem. 2009, 107, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Zaragosi, L.-E.; Dadone, B.; Michiels, J.-F.; Marty, M.; Pedeutour, F.; Dani, C.; Bianchini, L. Syndecan-1 regulates adipogenesis: New insights in dedifferentiated liposarcoma tumorigenesis. Carcinogenesis 2014, 36, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Landry, R.; Rioux, V.; Bensadoun, A. Characterization of syndecan-4 expression in 3T3-F442A mouse adipocytes: Link between syndecan-4 induction and cell proliferation. Cell Growth Differ. Mol. Boil. J. Am. Assoc. Cancer Res. 2001, 12, 497–504. [Google Scholar]
- Kalus, I.; Salmen, B.; Viebahn, C.; Von Figura, K.; Schmitz, D.; D’Hooge, R.; Dierks, T. Differential involvement of the extracellular 6-O-endosulfatases Sulf1 and Sulf2 in brain development and neuronal and behavioural plasticity. J. Cell. Mol. Med. 2008, 13, 4505–4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonewald, L.F.; Harris, S.E.; Rosser, J.; Dallas, M.R.; Dallas, S.L.; Camacho, N.P.; Boyan, B.; Boskey, A. Von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif. Tissue Int. 2003, 72, 537–547. [Google Scholar] [CrossRef]
- Kim, N.; Cho, S. Clinical applications of mesenchymal stem cells. Korean J. Intern. Med. 2013, 28, 387–402. [Google Scholar] [CrossRef]
- Najar, M.; Bouhtit, F.; Melki, R.; Afif, H.; Hamal, A.; Fahmi, H.; Merimi, M.; Lagneaux, L. Mesenchymal stromal cell-based therapy: New perspectives and challenges. J. Clin. Med. 2019, 8, 626. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, J. Bone tissue regeneration-application of mesenchymal stem cells and cellular and molecular mechanisms. Curr. Stem Cell Res. Ther. 2017, 12, 357–364. [Google Scholar] [CrossRef]
- Molténi, A.; Modrowski, D.; Hott, M.; Marie, P.J. Differential expression of fibroblast growth factor receptor-1, -2, and -3 and syndecan-1, -2, and -4 in neonatal rat mandibular condyle and calvaria during osteogenic differentiation in vitro. Bone 1999, 24, 337–347. [Google Scholar] [CrossRef]
- Scheideler, M.; Elabd, C.; Zaragosi, L.-E.; Chiellini, C.; Hackl, H.; Sánchez-Cabo, F.; Yadav, S.; Duszka, K.; Friedl, G.; Papak, C.; et al. Comparative transcriptomics of human multipotent stem cells during adipogenesis and osteoblastogenesis. BMC Genom. 2008, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, M.L.; Wang, Z.; Park, P.W.; Murphy, G.; Bernfield, M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a Timp-3-sensitive metalloproteinase. J. Cell Boil. 2000, 148, 811–824. [Google Scholar] [CrossRef]
- Szatmári, T.; Mundt, F.; Heidari-Hamedani, G.; Zong, F.; Ferolla, E.; Alexeyenko, A.; Hjerpe, A.; Dobra, K. Novel Genes and Pathways Modulated by Syndecan-1: Implications for the Proliferation and Cell-Cycle Regulation of Malignant Mesothelioma Cells. PLoS ONE 2012, 7, e48091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterfia, B.; Fule, T.; Baghy, K.; Szabadkai, K.; Fullár, A.; Dobos, K.; Zong, F.; Dobra, K.; Hollósi, P.; Jeney, A.; et al. Syndecan-1 enhances proliferation, migration and metastasis of HT-1080 cells in cooperation with syndecan-2. PLoS ONE 2012, 7, e39474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gresch, O.; Altrogge, L. Transfection of Difficult-to-Transfect Primary Mammalian Cells. Adv. Struct. Saf. Stud. 2011, 801, 65–74. [Google Scholar] [CrossRef]
- Marupanthorn, K.; Tantrawatpan, C.; Kheolamai, P.; Tantikanlayaporn, D.; Manochantr, S. Bone morphogenetic protein-2 enhances the osteogenic differentiation capacity of mesenchymal stromal cells derived from human bone marrow and umbilical cord. Int. J. Mol. Med. 2017, 39, 654–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lysdahl, H.; Baatrup, A.; Foldager, C.; Bünger, C. Preconditioning human mesenchymal stem cells with a low concentration of BMP2 stimulates proliferation and osteogenic differentiation in vitro. BioRes. Open Access 2014, 3, 278–285. [Google Scholar] [CrossRef]
- Almuraikhi, N.; Almasoud, N.; Binhamdan, S.; Younis, G.; Ali, D.; Manikandan, M.; Vishnubalaji, R.; Atteya, M.; Siyal, A.; Alfayez, M.; et al. Hedgehog signaling inhibition by smoothened antagonist BMS-833923 reduces osteoblast differentiation and ectopic bone formation of human skeletal (mesenchymal) stem cells. Stem Cells Int. 2019, 2019, 3435901–3435912. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhang, X.; Gu, R.; Liu, X.; Wang, S.; Xia, D.; Li, Z.; Lian, X.; Zhang, P.; Liu, Y.; et al. LAMA2 regulates the fate commitment of mesenchymal stem cells via hedgehog signaling. Stem Cell Res. Ther. 2020, 11, 135. [Google Scholar] [CrossRef]
- Wang, H.; Jin, H.; Rapraeger, A.C. Syndecan-1 and syndecan-4 capture epidermal growth factor receptor family members and the α3β1 integrin via binding sites in their ectodomains: Novel synstatins prevent kinase capture and inhibit α6β4-integrin-dependent epithelial cell motility. J. Biol. Chem. 2015, 290, 26103–26113. [Google Scholar] [CrossRef] [Green Version]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Boil. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Sugahara, K.; Kitagawa, H. Heparin and Heparan Sulfate Biosynthesis. IUBMB Life 2002, 54, 163–175. [Google Scholar] [CrossRef]
- Forsberg, M.; Holmborn, K.; Kundu, S.; Dagälv, A.; Kjellén, L.; Forsberg-Nilsson, K. Undersulfation of heparan sulfate restricts differentiation potential of mouse embryonic stem cells. J. Boil. Chem. 2012, 287, 10853–10862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menssen, A.; Häupl, T.; Sittinger, M.; Delorme, B.; Charbord, P.; Ringe, J. Differential gene expression profiling of human bone marrow-derived mesenchymal stem cells during adipogenic development. BMC Genom. 2011, 12, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaman, G.; Staines, K.A.; Farquharson, C.; Newton, P.T.; Dudhia, J.; Chenu, C.; Pitsillides, A.A.; Dhoot, G.K. Expression of Sulf1 and Sulf2 in cartilage, bone and endochondral fracture healing. Histochem. Cell Boil. 2015, 145, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Li, G.; Jiang, X. Fate determination in mesenchymal stem cells: A perspective from histone-modifying enzymes. Stem Cell Res. Ther. 2015, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, V.; Koo, C.Y.; Ibrahim, S.A.; Wang, Z.; Spillmann, D.; Dreier, R.; Kelsch, R.; Fischgräbe, J.; Smollich, M.; Rossi, L.H.; et al. Differential roles for membrane-bound and soluble syndecan-1 (CD138) in breast cancer progression. Carcinogenesis 2009, 30, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Zhong, D.; Xiao, Y.; Wang, B.; Wang, W.; Zhang, F.; Huang, H. Syndecan-1 knockdown inhibits glioma cell proliferation and invasion by deregulating a c-src/FAK-associated signaling pathway. Oncotarget 2017, 8, 40922–40934. [Google Scholar] [CrossRef]
- Okolicsanyi, R.K.; Camilleri, E.; Oikari, L.E.; Yu, C.; Cool, S.M.; Van Wijnen, A.J.; Griffiths, L.R.; Haupt, L.M. Human mesenchymal stem cells retain multilineage differentiation capacity including neural marker expression after extended in vitro expansion. PLoS ONE 2015, 10, e0137255. [Google Scholar] [CrossRef]
- Kuri-Harcuch, W.; Ramírez-Zacarías, J.L.; Castro-Munozledo, F. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with oil red O. Histochem. Cell Boil. 1992, 97, 493–497. [Google Scholar] [CrossRef]
- Oikari, L.E.; Okolicsanyi, R.K.; Qin, A.; Yu, C.; Griffiths, L.R.; Haupt, L.M. Cell surface heparan sulfate proteoglycans as novel markers of human neural stem cell fate determination. Stem Cell Res. 2016, 16, 92–104. [Google Scholar] [CrossRef] [Green Version]






| Accell siRNA | Sequences | |
|---|---|---|
| Non-targeting (Scrambled; SCR) | siRNA-1 | UGGUUUACAUGUCGACUAA |
| siRNA-2 | UGGUUUACAUGUUUUCUGA | |
| siRNA-3 | UGUUUACAUGUUUUCCUA | |
| siRNA-4 | UGGUUUACAUGUUGUGUGA | |
| SDC-1 | siRNA-1 | UGCUUAUUUGACAACGUUU |
| siRNA-2 | CUCUAGUUCUUUGUUCAUA | |
| siRNA-3 | GUGUUGUCUCUUGAGUUUG | |
| siRNA-4 | GGUUCAGCCAAGGUUUUAU |
| Gene Name | Symbol | Sequence | Amplicon (bp) | Ref Seq. | |
|---|---|---|---|---|---|
| BMP signalling | |||||
| Bone morphogenetic protein 2 | BMP2 | F | ACCCGCTGTCTTCTAGCGT | 180 | NM_001200 |
| R | TTTCAGGCCGAACATGCTGAG | ||||
| Bone morphogenetic protein 4 | BMP4 | F | ATGATTCCTGGTAACCGAATGC | 93 | NM_001202 |
| R | CCCCGTCTCAGGTATCAAACT | ||||
| Bone morphogenetic protein receptor type 1A | BMPR-IA | F | TGAAATCAGACTCCGACCAGA | 150 | NM_004329.2 |
| R | TGGCAAAGCAATGTCCATTAGTT | ||||
| Bone morphogenetic protein receptor type 1B | BMPR-IB | F | CTTTTGCGAAGTGCAGGAAAAT | 130 | NM_001256793.1 |
| R | TGTTGACTGAGTCTTCTGGACAA | ||||
| FGF signalling | |||||
| Fibroblast growth factor 2 | FGF2 | F | AAAAACGGGGGCTTCTTCCT | 86 | NM_002006.4 |
| R | TGTAGCTTGATGTGAGGGTCG | ||||
| Fibroblast growth factor receptor 1 | FGFR1 | F | CCGTATGTCCAGATCCTGAAGA | 126 | NM_001354368.1 |
| R | GATAGAGTTACCCGCCAAGCA | ||||
| Fibroblast growth factor receptor 2 | FGFR2 | F | TCAAGGTTCTCAAGCACTCGG | 89 | NM_022970.3 |
| R | ATATTCCCCAGCATCCGCCT | ||||
| Fibroblast growth factor receptor 3 | FGFR3 | F | CCCTACGTCACTGTACTCAAGACTG | 80 | NM_000142.5 |
| R | GTGACATTGTGCAAGGACAGAAC | ||||
| Wnt signalling | |||||
| Wnt family member 3A | Wnt3a | F | GTTGGGCCACAGTATTCCT | 111 | NM_033131.3 |
| R | GGGCATGATCTCCACGTAGT | ||||
| Frizzled-1 | FZD1 | F | ACCAACAGCAAACAAGGGGA | 163 | NM_003505.1 |
| R | GGAGCCTGCGAAAGAGAGTT | ||||
| SHH signalling | |||||
| Sonic hedgehog | SHH | F | TTATCCCCAATGTGGCCGAG | 168 | NM_000193.3 |
| R | TACACCTCTGAGTCATCAGCCT | ||||
| Patched-1 | PTCH1 | F | GGAGCAGATTTCCAAGGGGA | 128 | NM_001354918.1 |
| R | CCACAACCAAGAACTTGCCG | ||||
| Smoothened | SMO | F | TCAGCTGCCACTTCTACGAC | 83 | NM_005631.4 |
| R | CACATTGGCCTGACATAGCAC | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Peall, I.W.; Pham, S.H.; Okolicsanyi, R.K.; Griffiths, L.R.; Haupt, L.M. Syndecan-1 Facilitates the Human Mesenchymal Stem Cell Osteo-Adipogenic Balance. Int. J. Mol. Sci. 2020, 21, 3884. https://doi.org/10.3390/ijms21113884
Yu C, Peall IW, Pham SH, Okolicsanyi RK, Griffiths LR, Haupt LM. Syndecan-1 Facilitates the Human Mesenchymal Stem Cell Osteo-Adipogenic Balance. International Journal of Molecular Sciences. 2020; 21(11):3884. https://doi.org/10.3390/ijms21113884
Chicago/Turabian StyleYu, Chieh, Ian W. Peall, Son H. Pham, Rachel K. Okolicsanyi, Lyn R. Griffiths, and Larisa M. Haupt. 2020. "Syndecan-1 Facilitates the Human Mesenchymal Stem Cell Osteo-Adipogenic Balance" International Journal of Molecular Sciences 21, no. 11: 3884. https://doi.org/10.3390/ijms21113884
APA StyleYu, C., Peall, I. W., Pham, S. H., Okolicsanyi, R. K., Griffiths, L. R., & Haupt, L. M. (2020). Syndecan-1 Facilitates the Human Mesenchymal Stem Cell Osteo-Adipogenic Balance. International Journal of Molecular Sciences, 21(11), 3884. https://doi.org/10.3390/ijms21113884