Combination of Ipilimumab and Nivolumab in Cancers: From Clinical Practice to Ongoing Clinical Trials
Abstract
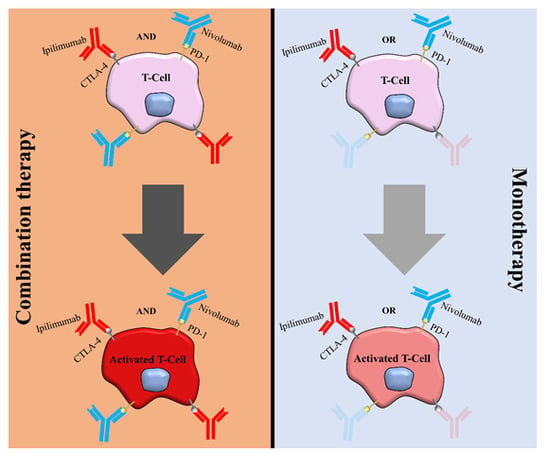
:1. Introduction
2. Methodology
3. Cytotoxic T Lymphocyte Antigen-4(CTLA-4)
4. Ipilimumab Pharmacology
5. Programmed Cell Death Protein 1 (PD-1)
6. Nivolumab Pharmacology
7. Combination Therapy in Different Cancers
7.1. Combination Therapy in Melanoma
7.2. Combination Therapy in Advanced Renal Cell Carcinoma
7.3. Combination Therapy in Colorectal Cancer
7.4. Combination Therapy in Breast Cancer
7.5. Combination Therapy in Lung Cancer
7.6. Combination Therapy in Esophageal Cancer
7.7. Combination Therapy in Hepatocellular Carcinoma
7.8. Combination Therapy in Hodgkin’sLymphoma
7.9. Combination Therapy in Head and Neck Cancer
7.10. Combination Therapy in Urothelial Carcinoma
7.11. Resistance to Immune Checkpoint Therapy
7.12. Immune-Related Adverse Events of Nivolumab and Ipilimumab
8. Expert Commentary
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ICIs | immune checkpoint inhibitors |
| CTLA-4 | cytotoxic T lymphocyte antigen-4 |
| PD-1 | programmed cell death protein 1 |
| FDA | the US food and drug administration |
| NSCLS | non-small cell lung cancer |
| SCLS | small cell lung cancer |
| RCC | renal cell carcinoma |
| HNC | head and neck cancer |
| UC | urothelial carcinoma |
| CRC | colorectal cancer |
| ORR | overall response rate |
| TKIs | tyrosine kinase inhibitors |
| STRs | short tandem repeats |
| MSI | microsatellite instability |
| MMR | mismatch repair |
| LD-SCLC | limited disease SCLC |
| ED-SCLC | extensive-disease SCLC |
| OS | overall survival |
| MDSCs | myeloid-derived suppressor cells |
| EC | esophageal cancer |
| HCC | hepatocellular carcinoma |
| MTD | maximum tolerated dose |
| CR | complete response |
| PR | partial response |
| mPFS | median progression-free survival |
| HNC | head and neck cancer |
| UC | urothelial carcinoma |
| UBC | urinary bladder cancer |
| UTUC | upper tract urothelial carcinoma |
| irAEs | immune-related adverse effects |
References
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, A. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashdown, M.L.; Robinson, A.P.; Yatomi-Clarke, S.L.; Ashdown, M.L.; Allison, A.; Abbott, D.; Markovic, S.N.; Coventry, B.J. Chemotherapy for Late-Stage Cancer Patients: Meta-Analysis of Complete Response Rates. F1000Research 2015, 4, 232. [Google Scholar] [CrossRef]
- Yu, J. Intestinal stem cell injury and protection during cancer therapy. Transl. Cancer Res. 2013, 2, 384–396. [Google Scholar] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Cushman, T.R.; Selek, U.; Tang, C.; Welsh, J.W. Safety of Combined Immunotherapy and Thoracic Radiation Therapy: Analysis of 3 Single-Institutional Phase I/II Trials. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1141–1148. [Google Scholar] [CrossRef]
- Palma, G.; Monti, S.; Xu, T.; Scifoni, E.; Yang, P.; Hahn, S.M.; Durante, M.; Mohan, R.; Liao, Z.; Cella, L. Spatial Dose Patterns Associated with Radiation Pneumonitis in a Randomized Trial Comparing Intensity-Modulated Photon Therapy with Passive Scattering Proton Therapy for Locally Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1124–1132. [Google Scholar] [CrossRef]
- Dess, R.T.; Sun, Y.; Matuszak, M.M.; Sun, G.; Soni, P.D.; Bazzi, L.; Murthy, V.L.; Hearn, J.W.D.; Kong, F.M.; Kalemkerian, G.P.; et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1395–1402. [Google Scholar] [CrossRef]
- Ferreira, E.B.; Ciol, M.A.; Vasques, C.I.; Bontempo Pde, S.; Vieira, N.N.; Silva, L.F.; Avelino, S.R.; Dos Santos, M.A.; Dos Reis, P.E. Gel of chamomile vs. urea cream to prevent acute radiation dermatitis in patients with head and neck cancer: A randomized controlled trial. J. Adv. Nurs. 2016, 72, 1926–1934. [Google Scholar] [CrossRef]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Lee, S.H.; Heo, Y.-S. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules 2019, 24, 1190. [Google Scholar] [CrossRef] [Green Version]
- Gadducci, A.; Guerrieri, M.E. Immune Checkpoint Inhibitors in Gynecological Cancers: Update of Literature and Perspectives of Clinical Research. Anticancer Res. 2017, 37, 5955–5965. [Google Scholar] [PubMed] [Green Version]
- Haanen, J.B.; Robert, C. Immune Checkpoint Inhibitors. Prog. Tumor Res. 2015, 42, 55–66. [Google Scholar] [PubMed] [Green Version]
- Khoja, L.; Butler, M.O.; Kang, S.P.; Ebbinghaus, S.; Joshua, A.M. Pembrolizumab. J. Immunother. Cancer 2015, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Ott, P.A.; Bang, Y.J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Mehnert, J.M.; Aung, K.L.; Lopez, J.; et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2535–2541. [Google Scholar] [CrossRef]
- Collin, M. Immune checkpoint inhibitors: A patent review (2010–2015). Expert Opin. Ther. Pat. 2016, 26, 555–564. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Chen, R.; Tao, Y.; Xu, X.; Shan, L.; Jiang, H.; Yin, Q.; Pei, L.; Cai, F.; Ma, L.; Yu, Y. The efficacy and safety of nivolumab, pembrolizumab, and atezolizumab in treatment of advanced non-small cell lung cancer. Discov. Med. 2018, 26, 155–166. [Google Scholar] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Massard, C.; Gordon, M.S.; Sharma, S.; Rafii, S.; Wainberg, Z.A.; Luke, J.; Curiel, T.J.; Colon-Otero, G.; Hamid, O.; Sanborn, R.E.; et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients with Advanced Urothelial Bladder Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.W.; Ardiani, A.; Farsaci, B.; Kwilas, A.R.; Gameiro, S.R. The tipping point for combination therapy: Cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin. Oncol. 2012, 39, 323–339. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunet, J.F.; Denizot, F.; Luciani, M.F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.G.; Golstein, P. A new member of the immunoglobulin superfamily--CTLA-4. Nature 1987, 328, 267–270. [Google Scholar] [CrossRef]
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Saverino, D.; Simone, R.; Bagnasco, M.; Pesce, G. The soluble CTLA-4 receptor and its role in autoimmune diseases: An update. Auto Immun. Highlights 2010, 1, 73–81. [Google Scholar] [CrossRef]
- Comin-Anduix, B.; Escuin-Ordinas, H.; Ibarrondo, F.J. Tremelimumab: Research and clinical development. Oncotargets Ther. 2016, 9, 1767–1776. [Google Scholar]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef]
- Calabrò, L.; Morra, A.; Giannarelli, D.; Amato, G.; D’Incecco, A.; Covre, A.; Lewis, A.; Rebelatto, M.C.; Danielli, R.; Altomonte, M.; et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): An open-label, non-randomised, phase 2 study. Lancet Respir. Med. 2018, 6, 451–460. [Google Scholar] [CrossRef]
- Antonia, S.; Goldberg, S.B.; Balmanoukian, A.; Chaft, J.E.; Sanborn, R.E.; Gupta, A.; Narwal, R.; Steele, K.; Gu, Y.; Karakunnel, J.J.; et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol. 2016, 17, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yang, W.; Huang, Y.; Cui, R.; Li, X.; Li, B. Evolving Roles for Targeting CTLA-4 in Cancer Immunotherapy. Cell. Physiol. Biochem. 2018, 47, 721–734. [Google Scholar] [CrossRef]
- Wróbel, S.; Przybyło, M.; Stępień, E. The Clinical Trial Landscape for Melanoma Therapies. J. Clin. Med. 2019, 8, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarhini, A.; Lo, E.; Minor, D.R. Releasing the brake on the immune system: Ipilimumab in melanoma and other tumors. Cancer Biother. Radiopharm. 2010, 25, 601–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourd, E. Nivolumab plus ipilimumab in metastatic colorectal cancer. Lancet Oncol. 2018, 19, e139. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- What is Yervoy and How is It Used? Available online: https://reference.medscape.com/drug/yervoy-ipilimumab-999636 (accessed on 25 February 2020).
- Sosa, A.; Lopez Cadena, E.; Simon Olive, C.; Karachaliou, N.; Rosell, R. Clinical assessment of immune-related adverse events. Ther. Adv. Med. Oncol. 2018, 10, 1758835918764628. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2017, 8, 2171–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eto, S.; Yoshikawa, K.; Nishi, M.; Higashijima, J.; Tokunaga, T.; Nakao, T.; Kashihara, H.; Takasu, C.; Iwata, T.; Shimada, M. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2016, 19, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Muenst, S.; Soysal, S.D.; Gao, F.; Obermann, E.C.; Oertli, D.; Gillanders, W.E. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2013, 139, 667–676. [Google Scholar] [CrossRef]
- Lipson, E.J.; Forde, P.M.; Hammers, H.J.; Emens, L.A.; Taube, J.M.; Topalian, S.L. Antagonists of PD-1 and PD-L1 in Cancer Treatment. Semin. Oncol. 2015, 42, 587–600. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhang, H.; Chen, B. Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J. Cancer 2017, 8, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Kazandjian, D.; Suzman, D.L.; Blumenthal, G.; Mushti, S.; He, K.; Libeg, M.; Keegan, P.; Pazdur, R. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer with Progression On or After Platinum-Based Chemotherapy. Oncologist 2016, 21, 634–642. [Google Scholar] [CrossRef] [Green Version]
- Raedler, L.A. Opdivo (Nivolumab): Second PD-1 Inhibitor Receives FDA Approval for Unresectable or Metastatic Melanoma. Am. Health Drug Benefits 2015, 8, 180–183. [Google Scholar]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Göppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [CrossRef]
- Hasan Ali, O.; Diem, S.; Markert, E.; Jochum, W.; Kerl, K.; French, L.E.; Speiser, D.E.; Früh, M.; Flatz, L. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. Oncoimmunology 2016, 5, e1231292. [Google Scholar] [CrossRef] [Green Version]
- Kopecký, J.; Kubecek, O.; Geryk, T.; Podhola, M.; Ziaran, M.; Priester, P.; Hanisova, M.; Borilova, S. Hepatic Injury Induced by a Single Dose of Nivolumab-a Case Report and Literature Review. Klin. Onkol. Cas. Ceske Slov. Onkol. Spol. 2019, 32, 133–138. [Google Scholar] [CrossRef]
- Rocha, M.; Correia de Sousa, J.; Salgado, M.; Araújo, A.; Pedroto, I. Management of Gastrointestinal Toxicity from Immune Checkpoint Inhibitor. GE Port. J. Gastroenterol. 2019, 26, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L.; Goldinger, S.M.; Hofmann, L.; Loquai, C.; Ugurel, S.; Thomas, I.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Göppner, D.; et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Bahia, J.; Mohebtash, M.; Barac, A. Cardiovascular Complications Associated with Novel Cancer Immunotherapies. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Marabondo, S.; Kaufman, H.L. High-dose interleukin-2 (IL-2) for the treatment of melanoma: Safety considerations and future directions. Expert Opin. Drug Saf. 2017, 16, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Zbytek, B.; Carlson, J.A.; Granese, J.; Ross, J.; Mihm, M.C., Jr.; Slominski, A. Current concepts of metastasis in melanoma. Expert Rev. Derm. 2008, 3, 569–585. [Google Scholar] [CrossRef] [Green Version]
- Diao, K.; Bian, S.X.; Routman, D.M.; Yu, C.; Ye, J.C.; Wagle, N.A.; Wong, M.K.; Zada, G.; Chang, E.L. Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: Clinical outcomes and toxicity. J. Neuro-Oncol. 2018, 139, 421–429. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [Green Version]
- Ather, M.H.; Masood, N.; Siddiqui, T. Current management of advanced and metastatic renal cell carcinoma. Urol. J. 2010, 7, 1–9. [Google Scholar]
- Escudier, B. Advanced renal cell carcinoma: Current and emerging management strategies. Drugs 2007, 67, 1257–1264. [Google Scholar] [CrossRef]
- Buckley, H.L.; Collinson, F.J.; Ainsworth, G.; Poad, H.; Flanagan, L.; Katona, E.; Howard, H.C.; Murden, G.; Banks, R.E.; Brown, J.; et al. PRISM protocol: A randomised phase II trial of nivolumab in combination with alternatively scheduled ipilimumab in first-line treatment of patients with advanced or metastatic renal cell carcinoma. BMC Cancer 2019, 19, 1102. [Google Scholar] [CrossRef]
- Yang, J.C.; Hughes, M.; Kammula, U.; Royal, R.; Sherry, R.M.; Topalian, S.L.; Suri, K.B.; Levy, C.; Allen, T.; Mavroukakis, S.; et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J. Immunother. 2007, 30, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Hammers, H.J.; Plimack, E.R.; Infante, J.R.; Rini, B.I.; McDermott, D.F.; Lewis, L.D.; Voss, M.H.; Sharma, P.; Pal, S.K.; Razak, A.R.A.; et al. Safety and Efficacy of Nivolumab in Combination with Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3851–3858. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Nojadeh, J.N.; Behrouz Sharif, S.; Sakhinia, E. Microsatellite instability in colorectal cancer. EXCLI J. 2018, 17, 159–168. [Google Scholar]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Morse, M.A.; Overman, M.J.; Hartman, L.; Khoukaz, T.; Brutcher, E.; Lenz, H.J.; Atasoy, A.; Shangguan, T.; Zhao, H.; El-Rayes, B. Safety of Nivolumab plus Low-Dose Ipilimumab in Previously Treated Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer. Oncologist 2019, 24, 1453–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastasiadi, Z.; Lianos, G.D.; Ignatiadou, E.; Harissis, H.V.; Mitsis, M. Breast cancer in young women: An overview. Updates Surg. 2017, 69, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Ataollahi, M.R.; Sharifi, J.; Paknahad, M.R.; Paknahad, A. Breast cancer and associated factors: A review. J. Med. Life 2015, 8, 6–11. [Google Scholar] [PubMed]
- Kolak, A.; Kamińska, M.; Sygit, K.; Budny, A.; Surdyka, D.; Kukiełka-Budny, B.; Burdan, F. Primary and secondary prevention of breast cancer. Ann. Agric. Environ. Med. AAEM 2017, 24, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.S.; Ready, N.; Healy, P.; Dumbauld, C.; Osborne, R.; Berry, M.; Shoemaker, D.; Clarke, J.; Crawford, J.; Tong, B.; et al. Immune Activation in Early-Stage Non-Small Cell Lung Cancer Patients Receiving Neoadjuvant Chemotherapy Plus Ipilimumab. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 7474–7482. [Google Scholar] [CrossRef] [Green Version]
- Ingold Heppner, B.; Untch, M.; Denkert, C.; Pfitzner, B.M.; Lederer, B.; Schmitt, W.; Eidtmann, H.; Fasching, P.A.; Tesch, H.; Solbach, C.; et al. Tumor-Infiltrating Lymphocytes: A Predictive and Prognostic Biomarker in Neoadjuvant-Treated HER2-Positive Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 5747–5754. [Google Scholar] [CrossRef] [Green Version]
- Asano, Y.; Kashiwagi, S.; Goto, W.; Takada, K.; Takahashi, K.; Morisaki, T.; Fujita, H.; Takashima, T.; Tomita, S.; Ohsawa, M.; et al. Prediction of treatment responses to neoadjuvant chemotherapy in triple-negative breast cancer by analysis of immune checkpoint protein expression. J. Transl. Med. 2018, 16, 87. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Alvarado-Luna, G.; Morales-Espinosa, D. Treatment for small cell lung cancer, where are we now?-a review. Transl. Lung Cancer Res. 2016, 5, 26–38. [Google Scholar]
- Pakkala, S.; Owonikoko, T.K. Immune checkpoint inhibitors in small cell lung cancer. J. Thorac. Dis. 2018, 10 (Suppl. 3), S460–S467. [Google Scholar] [CrossRef] [Green Version]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef] [Green Version]
- Reck, M.; Luft, A.; Szczesna, A.; Havel, L.; Kim, S.W.; Akerley, W.; Pietanza, M.C.; Wu, Y.L.; Zielinski, C.; Thomas, M.; et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 3740–3748. [Google Scholar] [CrossRef]
- Arriola, E.; Wheater, M.; Galea, I.; Cross, N.; Maishman, T.; Hamid, D.; Stanton, L.; Cave, J.; Geldart, T.; Mulatero, C.; et al. Outcome and Biomarker Analysis from a Multicenter Phase 2 Study of Ipilimumab in Combination with Carboplatin and Etoposide as First-Line Therapy for Extensive-Stage SCLC. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Jiang, Z. Advances in antibody therapeutics targeting small-cell lung cancer. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2018, 27, 1317–1323. [Google Scholar] [CrossRef] [Green Version]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheff, R.J.; Schneider, B.J. Non-small-cell lung cancer: Treatment of late stage disease: Chemotherapeutics and new frontiers. Semin. Interv. Radiol 2013, 30, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Govindan, R.; Szczesna, A.; Ahn, M.J.; Schneider, C.P.; Gonzalez Mella, P.F.; Barlesi, F.; Han, B.; Ganea, D.E.; Von Pawel, J.; Vladimirov, V.; et al. Phase III Trial of Ipilimumab Combined with Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Krasna, M. Overview of esophageal cancer. Ann. Cardiothorac. Surg. 2017, 6, 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janjigian, Y.Y.; Bendell, J.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Peltola, K.; Jaeger, D.; Evans, J.; et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients with Metastatic Esophagogastric Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Balogh, J.; Victor, D., III; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Qin, S. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Opportunities and Challenges. Oncologist 2019, 24 (Suppl. 1), S3–S10. [Google Scholar] [CrossRef] [Green Version]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Shanbhag, S.; Ambinder, R.F. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J. Clin. 2018, 68, 116–132. [Google Scholar] [CrossRef]
- Ansell, S.M. Hodgkin Lymphoma: Diagnosis and Treatment. Mayo Clin. Proc. 2015, 90, 1574–1583. [Google Scholar] [CrossRef] [Green Version]
- Matsuki, E.; Younes, A. Checkpoint Inhibitors and Other Immune Therapies for Hodgkin and Non-Hodgkin Lymphoma. Curr. Treat. Options Oncol. 2016, 17, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, W.; Linch, D. Hodgkin’s lymphoma in adults. Lancet 2012, 380, 836–847. [Google Scholar] [CrossRef] [Green Version]
- Herrera, A.F. Where does PD-1 blockade fit in HL therapy? Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Z.; Xiao, Y.; Zhu, X.-J.; Ye, C.; Zhou, J.-F. Anti-PD-1 therapy for clinical treatment of lymphoma: A single-arm meta-analysis. Oncotarget 2018, 9, 35343–35355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moy, R.H.; Younes, A. Immune Checkpoint Inhibition in Hodgkin Lymphoma. Hemasphere 2018, 2, e20. [Google Scholar] [CrossRef] [PubMed]
- Lo Nigro, C.; Denaro, N.; Merlotti, A.; Merlano, M. Head and neck cancer: Improving outcomes with a multidisciplinary approach. Cancer Manag. Res. 2017, 9, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Marur, S.; Forastiere, A.A. Head and neck cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef]
- Cognetti, D.M.; Weber, R.S.; Lai, S.Y. Head and neck cancer: An evolving treatment paradigm. Cancer 2008, 113 (Suppl. 7), 1911–1932. [Google Scholar] [CrossRef]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef]
- Pai, S.I.; Faivre, S.; Licitra, L.; Machiels, J.P.; Vermorken, J.B.; Bruzzi, P.; Gruenwald, V.; Giglio, R.E.; Leemans, C.R.; Seiwert, T.Y.; et al. Comparative analysis of the phase III clinical trials of anti-PD1 monotherapy in head and neck squamous cell carcinoma patients (CheckMate 141 and KEYNOTE 040). J. Immunother. Cancer 2019, 7, 96. [Google Scholar] [CrossRef]
- Miyazaki, J.; Nishiyama, H. Epidemiology of urothelial carcinoma. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2017, 24, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, B.; Srinivas, S. Urothelial carcinoma: The evolving landscape of immunotherapy for patients with advanced disease. Res. Rep. Urol. 2018, 10, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Seo, H.K. Immune checkpoint inhibitors for urothelial carcinoma. Investig. Clin. Urol. 2018, 59, 285–296. [Google Scholar] [CrossRef]
- Seront, E.; Catala, G.; Dermine, A.; Lejeune, S.; Rysselinck, S. Immune checkpoint inhibitors as a real hope in advanced urothelial carcinoma. Future Sci. OA 2018, 4, Fso341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Ready, N.; Farago, A.F.; de Braud, F.; Atmaca, A.; Hellmann, M.D.; Schneider, J.G.; Spigel, D.R.; Moreno, V.; Chau, I.; Hann, C.L.; et al. Third-Line Nivolumab Monotherapy in Recurrent SCLC: CheckMate 032. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohyama, C.; Kojima, T.; Kondo, T.; Naya, Y.; Inoue, T.; Tomita, Y.; Eto, M.; Hisasue, S.; Uemura, H.; Obara, W.; et al. Nivolumab in patients with unresectable locally advanced or metastatic urothelial carcinoma: CheckMate 275 2-year global and Japanese patient population analyses. Int. J. Clin. Oncol. 2019, 24, 1089–1098. [Google Scholar] [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 147–164. [Google Scholar] [CrossRef]
- Nowicki, T.S.; Hu-Lieskovan, S.; Ribas, A. Mechanisms of Resistance to PD-1 and PD-L1 Blockade. Cancer J. 2018, 24, 47–53. [Google Scholar] [CrossRef]
- Xia, A.; Zhang, Y.; Xu, J.; Yin, T.; Lu, X.-J. T Cell Dysfunction in Cancer Immunity and Immunotherapy. Front. Immunol. 2019, 10, 1719. [Google Scholar] [CrossRef] [Green Version]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef]
- Sanchez-Cespedes, M. B2M, JAK2 and MET in the genetic landscape of immunotolerance in lung cancer. Oncotarget 2018, 9, 35603–35604. [Google Scholar] [CrossRef] [PubMed]
- Yeon Yeon, S.; Jung, S.H.; Jo, Y.S.; Choi, E.J.; Kim, M.S.; Chung, Y.J.; Lee, S.H. Immune checkpoint blockade resistance-related B2M hotspot mutations in microsatellite-unstable colorectal carcinoma. Pathol. Res. Pract. 2019, 215, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Aptsiauri, N.; Doorduijn, E.M.; Garcia Lora, A.M.; van Hall, T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 2016, 39, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoyen-Ermis, D.; Tunali, G.; Tavukcuoglu, E.; Horzum, U.; Ozkazanc, D.; Sutlu, T.; Buyukasik, Y.; Esendagli, G. Myeloid maturation potentiates STAT3-mediated atypical IFN-γ signaling and upregulation of PD-1 ligands in AML and MDS. Sci. Rep. 2019, 9, 11697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, X. Primary and acquired resistance to PD-1/PD-L1 blockade in cancer treatment. Int. Immunopharmacol. 2017, 46, 210–219. [Google Scholar] [CrossRef]
- Lee, H.J.; Zhuang, G.; Cao, Y.; Du, P.; Kim, H.J.; Settleman, J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell 2014, 26, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Soundararajan, R.; Fradette, J.J.; Konen, J.M.; Moulder, S.; Zhang, X.; Gibbons, D.L.; Varadarajan, N.; Wistuba, I.I.; Tripathy, D.; Bernatchez, C.; et al. Targeting the Interplay between Epithelial-to-Mesenchymal-Transition and the Immune System for Effective Immunotherapy. Cancers 2019, 11, 714. [Google Scholar] [CrossRef] [Green Version]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef] [PubMed]
- Tobin, R.P.; Jordan, K.R.; Robinson, W.A.; Davis, D.; Borges, V.F.; Gonzalez, R.; Lewis, K.D.; McCarter, M.D. Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with Ipilimumab. Int. Immunopharmacol. 2018, 63, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Belum, V.R.; Benhuri, B.; Postow, M.A.; Hellmann, M.D.; Lesokhin, A.M.; Segal, N.H.; Motzer, R.J.; Wu, S.; Busam, K.J.; Wolchok, J.D.; et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur. J. Cancer 2016, 60, 12–25. [Google Scholar] [CrossRef] [Green Version]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29 (Suppl. 4), iv264–iv266. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., III; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [Green Version]
- Karamchandani, D.M.; Chetty, R. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: Pathologists’ perspective. J. Clin. Pathol. 2018, 71, 665–671. [Google Scholar] [CrossRef]
- Assarzadegan, N.; Montgomery, E.; Anders, R.A. Immune checkpoint inhibitor colitis: The flip side of the wonder drugs. Virchows Arch. Int. J. Pathol. 2018, 472, 125–133. [Google Scholar] [CrossRef]
- Joshi, M.N.; Whitelaw, B.C.; Palomar, M.T.; Wu, Y.; Carroll, P.V. Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: Clinical review. Clin. Endocrinol. 2016, 85, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sood, A.; Cole, D.; Abdollah, F.; Eilender, B.; Roumayah, Z.; Deebajah, M.; Dabaja, A.; Alanee, S. Endocrine, Sexual Function, and Infertility Side Effects of Immune Checkpoint Inhibitor Therapy for Genitourinary Cancers. Curr. Urol. Rep. 2018, 19, 68. [Google Scholar] [CrossRef]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated with Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorthy, R.S.; Moorthy, M.S.; Cunningham, E.T., Jr. Drug-induced uveitis. Curr. Opin. Ophthalmol. 2018, 29, 588–603. [Google Scholar] [CrossRef]
- Voskens, C.J.; Goldinger, S.M.; Loquai, C.; Robert, C.; Kaehler, K.C.; Berking, C.; Bergmann, T.; Bockmeyer, C.L.; Eigentler, T.; Fluck, M.; et al. The price of tumor control: An analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS ONE 2013, 8, e53745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadel, F.; El Karoui, K.; Knebelmann, B. Anti-CTLA4 antibody-induced lupus nephritis. N. Engl. J. Med. 2009, 361, 211–212. [Google Scholar] [CrossRef]
- Armand, P.; Nagler, A.; Weller, E.A.; Devine, S.M.; Avigan, D.E.; Chen, Y.B.; Kaminski, M.S.; Holland, H.K.; Winter, J.N.; Mason, J.R.; et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: Results of an international phase II trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 4199–4206. [Google Scholar] [CrossRef] [Green Version]
- Fecher, L.A.; Agarwala, S.S.; Hodi, F.S.; Weber, J.S. Ipilimumab and its toxicities: A multidisciplinary approach. Oncologist 2013, 18, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Warner, A.B.; Postow, M.A. Combination Controversies: Checkpoint Inhibition Alone or in Combination for the Treatment of Melanoma? Oncologist 2018, 32, 228–234. [Google Scholar]
- Trinh, S.; Le, A.; Gowani, S.; La-Beck, N.M. Management of Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitor Therapy: A Minireview of Current Clinical Guidelines. Asia Pac J. Oncol. Nurs. 2019, 6, 154–160. [Google Scholar]
- Ventola, C.L. Cancer Immunotherapy, Part 3: Challenges and Future Trends. Pharm. Ther. 2017, 42, 514–521. [Google Scholar]
- Baroudjian, B.; Arangalage, D.; Cuzzubbo, S.; Hervier, B.; Lebbé, C.; Lorillon, G.; Tazi, A.; Zalcman, G.; Bouattour, M.; Lioté, F.; et al. Management of immune-related adverse events resulting from immune checkpoint blockade. Expert Rev. Anticancer Ther. 2019, 19, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chaudhary, N.; Garg, M.; Floudas, C.S.; Soni, P.; Chandra, A.B. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Jin, G.; Pang, Y.; Huang, Y.; Wang, W.; Zhang, H.; Tuo, G.; Wu, P.; Wang, Z.; Zhu, Z. Comparative Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Advanced Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Choudhury, N.; Nakamura, Y. Importance of immunopharmacogenomics in cancer treatment: Patient selection and monitoring for immune checkpoint antibodies. Cancer Sci. 2016, 107, 107–115. [Google Scholar] [CrossRef]


| Reference | Trial Phase | Treatment Arms | Primary Endpoints | Results |
|---|---|---|---|---|
| [61] | 2 | Induction Phase: Nivolumab + Ipilimumab infusion (IV) Maintenance Phase: Nivolumab infusion (IV) | Intracranial CBR (up to six months) | The rate of intracranial CBR was 57% The rate of CR was 26%, the rate of PR was 30%, and the rate of SD for at least 6 months was 2%. |
| [62] | 3 | Arm A: Nivolumab+ Placebo for Ipilimumab+ Placebo for Nivolumab Arm B: Nivolumab+ Ipilimumab+ Placebo for Nivolumab | Rate of PFS Rate of OS (Time Frame: 6, 12, and 24 months) | The OS rate at 3 years was 58% in the nivolumab-plus-ipilimumab group and 52% in the nivolumab group, as compared with 34% in the ipilimumab group. |
| [63] | 2 | Cohort 1 Nivolumab Monotherapy (nivolumab 3 mg/kg every 2 weeks) Cohort 2 Nivolumab and Ipilimumab (nivolumab 1 mg/kg combined with ipilimumab 3 mg/kg every 3 weeks for four doses) | Intracranial response rate (at 3 years) | Intracranial responses were achieved by 20% of patients in cohort 1 and 46% of patients in cohort 2. Intracranial complete responses occurred in 12% of patients in cohort 1 and 17% of patients in cohort 2. |
| [64] | 3 | Arm A: Nivolumab+ Placebo for Ipilimumab+ Placebo for Nivolumab Arm B: Nivolumab+ Ipilimumab+ Placebo for Nivolumab | Rate of PFS Rate of OS(Time Frame: 6, 12, and 24 months) | The median OS was more than 60.0 months in the nivolumab-plus-ipilimumab group and 36.9 months in the nivolumab group, as compared with 19.9 months in the ipilimumab group. The OS at 5 years was 52% in the nivolumab-plus-ipilimumab group and 44% in the nivolumab group, as compared with 26% in the ipilimumab group. |
| [65] | 2 | Arm 1: Nivolumab (1 mg/kg+ Ipilimumab (3 mg/kg) Arm 2: Placebo + Ipilimumab | Percentage of participants with OR in the randomized, BRAF wild-type population (at a minimum of 6 months) | Among patients with BRAF wild-type tumors, the rate of OR was 61% in the combination group versus 11% in the ipilimumab-monotherapy group), with CR reported in 22% in the combination group and no patients in the ipilimumab-monotherapy group. |
| End Time | Trial Phase | Enrollment | Primary Endpoints | Treatment Arms | Clinical Trials Identifier |
|---|---|---|---|---|---|
| 2037 | 2 | 74 patients | ORR at two years | Nivolumab (240 mg every 2 weeks during the first 20 weeks, 480 mg every 4 weeks thereafter and Ipilimumab (After 2 weeks 1mg/kg every 6 weeks) | NCT03297593 |
| 2021 | 2 | 120 patients | PFS rate at one year | Arm A: Nivolumab (240mg and 360mg) Arm B: Nivolumab (3mg/kg) + Ipilimumab (1mg/kg) | NCT03117309 |
| 2021 | 2 | 53patients | Establish the recommended Phase II dose (RP2D) at 6 months ORR at two years | Entinostat (5mg, 3mg, or 2mg orally (PO) on D1, 8, 15), Nivolumab (3 mg/kg IV D1 and Ipilimumab 1 mg/kg IV D1) | NCT03552380 |
| 2024 | 3 | 676patients | Duration of PFS (Time Frame: up to 23 months) | Experimental Arm: Cabozantinib + nivolumab + ipilimumab (4 doses) followed by cabozantinib + nivolumab Control Arm: Cabozantinib-matched placebo + nivolumab + ipilimumab (4 doses) followed by cabozantinib-matched placebo + nivolumab | NCT03937219 |
| End Time | Enrollment | Trial Phase | Primary Endpoints | Treatment Arms | Clinical Trials Identifier |
|---|---|---|---|---|---|
| 2021 | 32 | 1 | To determine the recommended dose level of the combination of regorafenib, nivolumab, and ipilimumab in patients with advanced metastatic RCC | Patients receive regorafenib on days 1–21, nivolumab, and ipilimumab IV. Cycles repeat every 28 days for up to 2 years | NCT04362839 |
| 2022 | 100 | 2 | The 8-month PFS rate | Temozolomide 150 mg/sqm daily on days 1–5 every 4 weeks, for two cycles followed by TC scan assessment, nivolumab 480 mg i.v. every 4 weeks, low-dose ipilimumab 1 mg/Kg i.v. every 8 weeks and temozolomide | NCT03832621 |
| 2025 | 494 | 3 | PFS (Time Frame: Up to 5 years) | Arm A: Nivolumab Monotherapy Arm B: Nivolumab + Ipilimumab Combination Arm C: Investigator’s Choice Chemotherapy | NCT04008030 |
| 2024 | 80 | 2 | Disease control rate (Time Frame: 2 years) | Nivolumab (3 times per cycle) +Ipilimumab (once per cycle) Radiation Therapy | NCT03104439 |
| Estimated time | Enrollment | Trial Phase | Primary Endpoints | Treatment Arms | Clinical Trial Identifier |
|---|---|---|---|---|---|
| 2017–2022 | 21 participants | Phase 1/2 | PFS (Time Frame: 6 months) | Thoracic Radiation Therapy (3Gy × 10 fractions) for 10 days Ipilimumab 3 mg/kg (90 min IV infusion) every 3 weeks plus Nivolumab 1 mg/kg (30 min IV infusion) will be administered every 3 weeks | NCT03043599 |
| 2018–2021 | 41 participants | Phase 2 | Disease Control Rate (DCR) (TimeFrame: up to 3 years) | Combination immunotherapy with Ipilimumab and Nivolumab plus a Dendritic Cell-based p53 Vaccine (Ad.p53-DC) | NCT03406715 |
| 2014–2022 | 264 participants | Phase 2 | The OS and PFS rates (at a maximum of 6,5 years) | Induction: Nivolumab at a dose of 1 mg/kg i.v. followed (on the same day) by Ipilimumab at a dose of 3 mg/kg i.v. once every 3 weeks, 4 cycles Maintenance: Nivolumab 240 mg i.v. once every 2 weeks, for a maximum of 12 months from the start of maintenance | NCT02046733 |
| 2018–2022 | 55 participants | Phase 1/2 | Phase I: Maximum tolerated dose (MTD) (Time Frame: 9 Months) Phase II: PFS (Time Frame: 36 Months) | Phase I: nivolumab, ipilimumab, and plinabulin Phase II Arm A: nivolumab and ipilimumab Phase II Arm B: nivolumab, ipilimumab, and plinabulin | NCT03575793 |
| End Time | Enrollment | Trial Phase | Primary Endpoints | Treatment Arms | Clinical Trial Identifier |
|---|---|---|---|---|---|
| 2020 | 184 | 2 | The ORR at two years | Arm 1: Nivolumab (3 mg/kg, every two weeks) Arm 2: Nivolumab (3 mg/kg, every two weeks) and Ipilimumab (1 mg/kg, every six weeks) | NCT03091491 |
| 2020 | 472 | 1 | Number of participants who experienced serious adverse events and adverse events, the number of participants who experienced selected adverse Events, and the number of participants with abnormalities in selected hepatic and thyroid clinical laboratory tests | Nivolumab in combination with Gemcitabine, Cisplatin, Pemetrexed, Paclitaxel, Carboplatin, Bevacizumab, Erlotinib, and Ipilimumab in different arms | NCT01454102 |
| 2025 | 580 | 3 | PFS (Time Frame: up to 47 months) | Arm 1: Nivolumab+Platinum doublet chemotherapy Arm 2: Nivolumab + Ipilimumab Arm 3: Platinum doublet chemotherapy | NCT02864251 |
| End Time | Enrollment | Phase | Primary Endpoints | Treatment Arms | Clinical Trial Identifier |
|---|---|---|---|---|---|
| 2022 | 130 | 2 | 12-months PFS | Arm A: Chemoradiation (50Gy in 25 fractions over 5 weeks (i.e., 2Gy per fraction), concurrently with 3 cycles of 2 weeks of FOLFOX) + Nivolumab (IV 240 mg on days 1, 15 and 29) Arm B: Chemoradiation + Nivolumab + Ipilimumab (IV 1 mg/kg on day 1 followed by a maintenance phase) | NCT03437200 |
| 2021 | 939 | 3 | OS and PFS | Arm A: Nivolumab + Ipilimumab Arm B: Nivolumab + Cisplatin + Fluorouacil Arm C: Cisplatin + Fluorouracil | NCT03143153 |
| 2021 | 75 | 2 | OS (Time Frame:36 months) | Arm A: Nivolumab/Ipilimumab combination treatment B. Nivolumab monotherapy | NCT03416244 |
| 2023 | 278 | 2/3 | Pathologic CR (Step I) (Time Frame: Up to 5 weeks) Disease-free survival (DFS) (Step 2) | Arm A (carboplatin, paclitaxel, radiation therapy) Arm B (carboplatin, paclitaxel, radiation therapy, nivolumab) Arm C (nivolumab) Arm D (nivolumab, ipilimumab) | NCT03604991 |
| 2022 | 97 | 2 | OS (at 12 months) | Arm A: Chemo-free immunotherapy with Nivolumab, Ipilimumab, Trastuzumab Arm B: Addition of Nivolumab to Standard therapy (chemotherapy and Trastuzumab) | NCT03409848 |
| End Time | Enrollment | Trial Phase | Primary Endpoints | Treatment Arms | Clinical Trial Identifier |
|---|---|---|---|---|---|
| 2022 | 32 | 1/2 | Delay to surgery (Time Frame: Up to Day 89) Incidence of treatment-emergent adverse events (Time Frame: Up to Day 127) | Ipilimumab (1 mg/kg, once every 3 weeks, for 3 weeks) + Nivolumab (3 mg/kg, once every 3 weeks, for 6 weeks) | NCT03682276 |
| 2023 | 1084 | 3 | OS (Time Frame: up to 4 years) | Arm A: Nivolumab + Ipilimumab Arm B: Sorafenib/Lenvatinib | NCT04039607 |
| 2024 | 12 | 1 | Drug-related toxicities (Time Frame: 4 years) Fold change in interferon-producing DNAJB1-PRKACA-specific cluster of differentiation 8(CD8) and 4 (CF4) T cells | DNAJB1-PRKACA peptide vaccine, Nivolumab, and Ipilimumab | NCT04248569 |
| 2022 | 1097 | 1/2 | Safety and Tolerability of nivolumab ORR, Safety, and Tolerability of nivolumab plus ipilimumab | Non-infected: Nivolumab HCV-infected: Nivolumab HBV-infected: Nivolumab Nivolumab plus Ipilimumab Combination Nivolumab plus Cabozantinib Combination Nivolumab plus Ipilimumab plus Cabozantinib | NCT01658878 |
| 2022 | 32 | 1/2 | Ipilimumab Nivolumab | NCT03682276 |
| End Time | Enrollment | Trial Phase | Primary Endpoints | Treatment Arms | Clinical Trial Identifier |
|---|---|---|---|---|---|
| 2022 | 24 | 1 | Incidence of adverse events (Time Frame: Up to 6 months) | Nivolumab Ipilimumab Radiation Therapy | NCT03162731 |
| 2024 | 60 | 2 | Adverse Events related to treatment (Time Frame: Up to 4 months) | Arm A: Nivolumab + Relatlimab Arm B: Nivolumab + Ipilimumab Arm C: Nivolumab | NCT04080804 |
| 2024 | 140 | 2 | 2 years of disease-free survival | Arm A: Nivolumab Arm B: Ipilimumab+ Nivolumab | NCT03406247 |
| 2024 | 40 | 2 | Response rates to treatment (Time Frame: at time of surgery) | Arm A: Nivolumab Arm B: Ipilimumab+ Nivolumab | NCT02919683 |
| 2020 | 36 | 1 | Change in immune profile in the tumor microenvironment Change in circulating percentage of immune suppressor subsets in peripheral blood Phenotypic shifts in T cell subsets in peripheral blood | Group A (VX15/2503) Group B (VX15/2503, ipilimumab) Group C (VX15/2503, nivolumab) Group D (nivolumab) Group E (ipilimumab) | NCT03690986 |
| 2026 | 947 | 3 | OS in participants with PD-L1 expressing tumors. (Time Frame: Approximately 51 months) OS in all participants | Experimental: Nivolumab and Ipilimumab Active Comparator: Extreme Regimen | NCT02741570 |
| 2024 | 675 | 2 | ORR in the platinum-refractory subgroup (Time Frame: 28 months) Duration of response in the platinum-refractory subgroup (Time Frame: 28 months) | Experimental: Nivolumab and Ipilimumab Active Comparator: Nivolumab and Ipilimumab-placebo | NCT02823574 |
| 2024 | 276 | 3 | Disease-free survival (Time Frame: approximately 71 months) | Experimental: Neoadjuvant/adjuvant Nivolumab and Ipilimumab Active Comparator: Surgical resection + adjuvant radio(-chemo) therapy | NCT03700905 |
| 2021 | 32 | 1/2 | The number of patients that will not endure a delay in surgery Tumor response to NAI The potential impact of local tumor hypoxia on tumor T cell abundance (Time Frame: 2.5 years) | Experimental: Nivolumab with or without Ipilimumab | NCT03003637 |
| Primary Resistance | Reference | Secondary Resistance | Reference |
|---|---|---|---|
| Presence of inactivating mutations in JAK1, JAK2, and beta2-microglobulin (B2M) | [122] | Inactivating mutations in beta2-microglobulin (B2M) | [123] |
| Lower MHC-I expression | [124] | Increased PD-L2 expression on PD-L1 negative tumor cells | [125] |
| Overexpression of VEGF | [126] | PD-L1 up-regulation | [127] |
| Activation of PI3K/AKT, ALK/STAT3, and MEK/ERK/STAT1 signaling pathways | [128] | JAK1/2 mutation | [129] |
| TGF-β signaling pathway | [130] | ||
| Epithelial-mesenchymal transition (EMT) | [131] | ||
| Exhaustion of T cells | [132] | ||
| Increase in Tumor-associated macrophage and Myeloid-derived suppressor cells (MDSCs) | [133] |
| Common irAEs | CTLA-4 Inhibitors | PD-1 Inhibitors | Combination of Nivolumab and Ipilimumab |
|---|---|---|---|
| Cutaneous | |||
| Rash | 34% | 10–21% | 30% |
| Pruritus | 25–30% | 11–21% | 35% |
| Vitiligo | 4% | 11% | 9% |
| Gastrointestinal Disease | |||
| Diarrhea | 38% | 8–20% | 45% |
| Colitis | 8–10% | 1–3% | 13% |
| Neurological Disease | 4% | 6% | 12% |
| Endocrine system | |||
| Hypothyroidism | 1–2% | 4–10% | 17% |
| Hyperthyroidism | 2–3% | Less than 1% | 7% |
| Lung | |||
| Pneumonitis | Less than 1% | 1–5% | 7% |
| Liver | |||
| Hepatitis | Less than 1% | 1–2% | 14–18% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kooshkaki, O.; Derakhshani, A.; Hosseinkhani, N.; Torabi, M.; Safaei, S.; Brunetti, O.; Racanelli, V.; Silvestris, N.; Baradaran, B. Combination of Ipilimumab and Nivolumab in Cancers: From Clinical Practice to Ongoing Clinical Trials. Int. J. Mol. Sci. 2020, 21, 4427. https://doi.org/10.3390/ijms21124427
Kooshkaki O, Derakhshani A, Hosseinkhani N, Torabi M, Safaei S, Brunetti O, Racanelli V, Silvestris N, Baradaran B. Combination of Ipilimumab and Nivolumab in Cancers: From Clinical Practice to Ongoing Clinical Trials. International Journal of Molecular Sciences. 2020; 21(12):4427. https://doi.org/10.3390/ijms21124427
Chicago/Turabian StyleKooshkaki, Omid, Afshin Derakhshani, Negar Hosseinkhani, Mitra Torabi, Sahar Safaei, Oronzo Brunetti, Vito Racanelli, Nicola Silvestris, and Behzad Baradaran. 2020. "Combination of Ipilimumab and Nivolumab in Cancers: From Clinical Practice to Ongoing Clinical Trials" International Journal of Molecular Sciences 21, no. 12: 4427. https://doi.org/10.3390/ijms21124427
APA StyleKooshkaki, O., Derakhshani, A., Hosseinkhani, N., Torabi, M., Safaei, S., Brunetti, O., Racanelli, V., Silvestris, N., & Baradaran, B. (2020). Combination of Ipilimumab and Nivolumab in Cancers: From Clinical Practice to Ongoing Clinical Trials. International Journal of Molecular Sciences, 21(12), 4427. https://doi.org/10.3390/ijms21124427