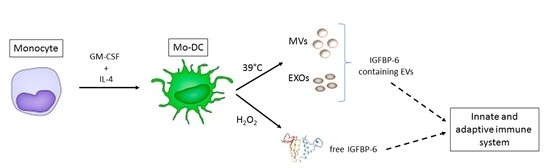
Insulin-Like Growth Factor Binding Protein 6 Is Secreted in Extracellular Vesicles upon Hyperthermia and Oxidative Stress in Dendritic Cells But Not in Monocytes
Abstract
:1. Introduction
2. Results
2.1. IGFBP-6 Is Found Associated with Microvesicles and Exosomes from DCs upon Hyperthermia Exposure
2.2. IGFBP-6 Is Secreted by Stimulated DCs
3. Discussion
4. Materials and Methods
4.1. Generation of Monocyte-Derived Dendritic Cells
4.2. Isolation and Characterization of Microvesicles and Exosomes
4.3. Analysis of IGFBP-6 Protein in Conditioned Medium and Cell Lysates
4.4. Western Blotting Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EXOs | Exosomes |
| Mo-DCs | Monocyte-derived dendritic cells |
| MVs | Microvesicles |
References
- Bach, L.A. IGFBP-6 five years on; not so ‘forgotten’? Growth Horm. IGF Res. 2005, 15, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.A. Current ideas on the biology of IGFBP-6: More than an IGF-II inhibitor? Growth Horm. IGF Res. 2016, 30–31, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.A. Recent insights into the actions of IGFBP-6. J. Cell Commun. Signal. 2015, 9, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liso, A.; Capitanio, N.; Gerli, R.; Conese, M. From fever to immunity: A new role for IGFBP-6? J. Cell. Mol. Med. 2018, 22, 4588–4596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liso, A.; Castellani, S.; Massenzio, F.; Trotta, R.; Pucciarini, A.; Bigerna, B.; De Luca, P.; Zoppoli, P.; Castiglione, F.; Palumbo, M.C.; et al. Human monocyte-derived dendritic cells exposed to hyperthermia show a distinct gene expression profile and selective upregulation of IGFBP6. Oncotarget 2017, 8, 60826. [Google Scholar] [CrossRef] [Green Version]
- Conese, M.; D’Oria, S.; Castellani, S.; Trotta, R.; Montemurro, P.; Liso, A. Insulin-like growth factor-6 (IGFBP-6) stimulates neutrophil oxidative burst, degranulation and chemotaxis. Inflamm. Res. 2018, 67, 107–109. [Google Scholar] [CrossRef]
- Alunno, A.; Bistoni, O.; Manetti, M.; Cafaro, G.; Valentini, V.; Bartoloni, E.; Gerli, R.; Liso, A. Insulin-Like Growth Factor Binding Protein 6 in Rheumatoid Arthritis: A Possible Novel Chemotactic Factor? Front. Immunol. 2017, 8, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Pol, E.; Boing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Lindenbergh, M.F.S.; Stoorvogel, W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Tsaprailis, G.; Chen, Q.M. Proteomic identification of insulin-like growth factor-binding protein-6 induced by sublethal H2O2 stress from human diploid fibroblasts. Mol. Cell. Proteom. 2005, 4, 1273–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savina, A.; Amigorena, S. Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 2007, 219, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Sobenin, I.A.; Orekhov, A.N.; Bobryshev, Y.V. Dendritic cells in atherosclerotic inflammation: The complexity of functions and the peculiarities of pathophysiological effects. Front. Physiol. 2014, 5, 196. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [Green Version]
- Giusti, I.; Di Francesco, M.; Cantone, L.; D’Ascenzo, S.; Bollati, V.; Carta, G.; Dolo, V. Time-dependent release of extracellular vesicle subpopulations in tumor CABA I cells. Oncol. Rep. 2015, 34, 2752–2759. [Google Scholar] [CrossRef] [Green Version]
- Lancaster, G.I.; Febbraio, M.A. Exosome-dependent trafficking of HSP70: A novel secretory pathway for cellular stress proteins. J. Biol. Chem. 2005, 280, 23349–23355. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, A. Not all extracellular vesicles were created equal: Clinical implications. Ann. Transl. Med. 2017, 5, 111. [Google Scholar] [CrossRef] [Green Version]
- Benites, B.D.; Alvarez, M.C.; Saad, S.T.O. Small Particles, Big Effects: The Interplay between Exosomes and Dendritic Cells in Antitumor Immunity and Immunotherapy. Cells 2019, 8, 1648. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Grechko, A.V.; Orekhov, A.N.; Bobryshev, Y.V. An immunoregulatory role of dendritic cell-derived exosomes versus HIV-1 infection: Take it easy but be warned. Ann. Transl. Med. 2017, 5, 362. [Google Scholar] [CrossRef] [Green Version]
- Thery, C.; Boussac, M.; Veron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef] [Green Version]
- Bucio-Lopez, L.; Pinon-Zarate, G.; Jarquin-Yanez, K.; Hernandez-Tellez, B.; Herrera-Henriquez, M.A.; Castell-Rodriquez, A.E. Phenotype of exosomes derived from dendritic cells tretaed with different stimuli. J. Immunol. Clin. Res. 2018, 5, 1046. [Google Scholar]
- Chambery, D.; de Galle, B.; Babajko, S. Retinoic acid stimulates IGF binding protein (IGFBP)-6 and depresses IGFBP-2 and IGFBP-4 in SK-N-SH human neuroblastoma cells. J. Endocrinol. 1998, 159, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.Y.; Guo, L.; Zhao, X.J.; Liu, H.; Lei, T.; Ma, D.J.; Gao, X.Y. Transcriptional activation of insulin-like growth factor binding protein 6 by 17beta-estradiol in SaOS-2 cells. Exp. Mol. Med. 2009, 41, 478–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, L.A.; Fu, P.; Yang, Z. Insulin-like growth factor-binding protein-6 and cancer. Clin. Sci. 2013, 124, 215–229. [Google Scholar] [CrossRef] [Green Version]
- Osugi, Y.; Vuckovic, S.; Hart, D.N. Myeloid blood CD11c(+) dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood 2002, 100, 2858–2866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefford, M.; Schnurr, M.; Toy, T.; Masterman, K.A.; Shin, A.; Beecroft, T.; Tai, T.Y.; Shortman, K.; Shackleton, M.; Davis, I.D.; et al. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: Differential regulation of function by specific classes of physiologic stimuli. Blood 2003, 102, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Andersson, L.I.; Cirkic, E.; Hellman, P.; Eriksson, H. Myeloid blood dendritic cells and monocyte-derived dendritic cells differ in their endocytosing capability. Hum. Immunol. 2012, 73, 1073–1081. [Google Scholar] [CrossRef]
- Wimmers, F.; Schreibelt, G.; Skold, A.E.; Figdor, C.G.; De Vries, I.J. Paradigm Shift in Dendritic Cell-Based Immunotherapy: From in vitro Generated Monocyte-Derived DCs to Naturally Circulating DC Subsets. Front. Immunol. 2014, 5, 165. [Google Scholar] [CrossRef]
- Van Leeuwen-Kerkhoff, N.; Lundberg, K.; Westers, T.M.; Kordasti, S.; Bontkes, H.J.; Lindstedt, M.; de Gruijl, T.D.; van de Loosdrecht, A.A. Human Bone Marrow-Derived Myeloid Dendritic Cells Show an Immature Transcriptional and Functional Profile Compared to Their Peripheral Blood Counterparts and Separate from Slan+ Non-Classical Monocytes. Front. Immunol. 2018, 9, 1619. [Google Scholar] [CrossRef]



| Sample | Dimension(nm) | PDI | Z-Potential (mV) |
|---|---|---|---|
| MVs 37 °C 3 h | 369 ± 25 | 0.32 | −12.1 ± 0.4 |
| MVs 39 °C 3 h | 294 ± 22 | 0.38 | −12.7 ± 0.3 |
| EXOs 37 °C 3 h | 105 ± 7 | 0.52 | −11.0 ± 2.1 |
| EXOs 39 °C 3 h | 119 ± 11 | 0.49 | −9.5 ± 1.2 |
| MVs 37 °C 24 h | 291 ± 7 | 0.29 | −12.4 ± 0.6 |
| MVs 39 °C 24 h | 332 ± 17 | 0.41 | −12.1 ± 0.3 |
| EXOs 37 °C 24 h | 94 ± 8 | 0.34 | −10.3 ± 0.8 |
| EXOs 39 °C 24 h | 121 ± 13 | 0.46 | 9.2 ± 0.4 |
| MVs 37 °C 48 h | 298 ± 17 | 0.28 | −12.0 ± 1.0 |
| MVs 39 °C 48 h | 272 ± 15 | 0.36 | −11.7 ± 0.4 |
| EXOs 37 °C 48 h | 106 ± 13 | 0.36 | −9.5 ± 0.8 |
| EXOs 39 °C 48 h | 169 ± 7 | 0.41 | −8.5 ± 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conese, M.; Pace, L.; Pignataro, N.; Catucci, L.; Ambrosi, A.; Di Gioia, S.; Tartaglia, N.; Liso, A. Insulin-Like Growth Factor Binding Protein 6 Is Secreted in Extracellular Vesicles upon Hyperthermia and Oxidative Stress in Dendritic Cells But Not in Monocytes. Int. J. Mol. Sci. 2020, 21, 4428. https://doi.org/10.3390/ijms21124428
Conese M, Pace L, Pignataro N, Catucci L, Ambrosi A, Di Gioia S, Tartaglia N, Liso A. Insulin-Like Growth Factor Binding Protein 6 Is Secreted in Extracellular Vesicles upon Hyperthermia and Oxidative Stress in Dendritic Cells But Not in Monocytes. International Journal of Molecular Sciences. 2020; 21(12):4428. https://doi.org/10.3390/ijms21124428
Chicago/Turabian StyleConese, Massimo, Lorenzo Pace, Nicoletta Pignataro, Lucia Catucci, Antonio Ambrosi, Sante Di Gioia, Nicola Tartaglia, and Arcangelo Liso. 2020. "Insulin-Like Growth Factor Binding Protein 6 Is Secreted in Extracellular Vesicles upon Hyperthermia and Oxidative Stress in Dendritic Cells But Not in Monocytes" International Journal of Molecular Sciences 21, no. 12: 4428. https://doi.org/10.3390/ijms21124428
APA StyleConese, M., Pace, L., Pignataro, N., Catucci, L., Ambrosi, A., Di Gioia, S., Tartaglia, N., & Liso, A. (2020). Insulin-Like Growth Factor Binding Protein 6 Is Secreted in Extracellular Vesicles upon Hyperthermia and Oxidative Stress in Dendritic Cells But Not in Monocytes. International Journal of Molecular Sciences, 21(12), 4428. https://doi.org/10.3390/ijms21124428