The Protective Effect of Chlorogenic Acid on Vascular Senescence via the Nrf2/HO-1 Pathway
Abstract
:1. Introduction
2. Results
2.1. CGA Inhibits AngII-Induced Vascular Senescence
2.2. Treatment with CGA Attenuates H2O2-Induced Cellular Senescence in HUVECs
2.3. CGA Exerts a Favorable Effect on Senescence-Related Markers
2.4. CGA Induces Nrf2 and HO-1 Expression
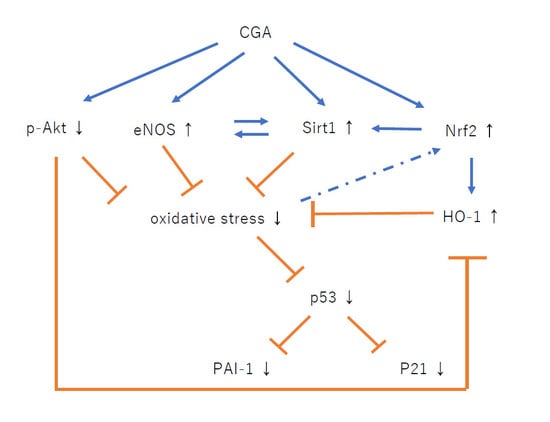
2.5. CGA Attenuates Senescence of Vascular Endothelial Cells through the Nrf2/HO-1 Pathway
3. Discussion
4. Materials and Methods
4.1. Mice and Study Protocol
4.2. Blood Pressure Measurement
4.3. SA-β-Gal Staining
4.4. Cell Culture and Treatment
4.5. Cellular Senescence
4.6. Cell Viability
4.7. Western Blotting
4.8. Real-Time Polymerase Chain Reaction
4.9. Reagents
4.10. Treatment with ZnPP In Vivo
4.11. Treatment with ZnPP In Vitro
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EC | Endothelial cell |
| AngII | Angiotensin II |
| CGA | Chlorogenic acids |
| Nrf2 | Nuclear factor erythroid 2-factor 2 |
| HO-1 | Heme Oxygenase-1 |
| SBP | Systolic blood pressure |
| SA-β-gal | Senescence associated-β-galactosidase |
| HUVEC | Human Umbilical Vein Endothelial Cell |
| 8-OHdG | 8-hydroxy-2’-deoxyguanosine |
| Sirt1 | Sirtuin 1 |
| eNOS | endothelial nitric oxide synthase |
| PAI-1 | Plasminogen activator inhibitor-1 |
| ZnPP | Zinc protoporphyrin IX |
| keap1 | kelch-like ECH-associated protein 1 |
| SOD1 | Superoxide dismutase 1 |
| CAT | Catalase |
| NQO1 | NAD(P)H dehydrogenase [quinone] 1 |
| GCLC | Glutamate-cysteine ligase catalytic subunit |
| GCLM | Glutamate-cysteine ligase modifier subunit |
References
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.; Feletou, M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbich, C.; Dimmeler, S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ. Res. 2004, 95, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Xia, S.; Kalionis, B.; Wan, W.; Sun, T. The role of oxidative stress and inflammation in cardiovascular aging. BioMed Res. Int. 2014, 2014, 615312. [Google Scholar] [CrossRef] [PubMed]
- Ghebre, Y.T.; Yakubov, E.; Wong, W.T.; Krishnamurthy, P.; Sayed, N.; Sikora, A.G.; Bonnen, M.D. Vascular Aging: Implications for Cardiovascular Disease and Therapy. Transl. Med. (Sunnyvale) 2016, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, metabolism and cardiovascular disease. J. Physiol. 2016, 594, 2061–2073. [Google Scholar] [CrossRef]
- Naylor, R.M.; Baker, D.J.; van Deursen, J.M. Senescent cells: A novel therapeutic target for aging and age-related diseases. Clin. Pharmacol. Ther. 2013, 93, 105–116. [Google Scholar] [CrossRef]
- Park, S.Y.; Kwon, O.S.; Andtbacka, R.H.I.; Hyngstrom, J.R.; Reese, V.; Murphy, M.P.; Richardson, R.S. Age-related endothelial dysfunction in human skeletal muscle feed arteries: The role of free radicals derived from mitochondria in the vasculature. Acta Physiol. 2018, 222, e12893. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Piao, S.; Lee, I.; Nagar, H.; Choi, S.J.; Shin, N.; Kim, D.W.; Shong, M.; Jeon, B.H.; Kim, C.S. CR6 interacting factor 1 deficiency induces premature senescence via SIRT3 inhibition in endothelial cells. Free Radic. Biol. Med. 2020, 150, 161–171. [Google Scholar] [CrossRef]
- Schosserer, M.; Grillari, J.; Breitenbach, M. The Dual Role of Cellular Senescence in Developing Tumors and Their Response to Cancer Therapy. Front. Oncol. 2017, 7, 278. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.; San Hipolito-Luengo, A.; Villalobos, L.A.; Vallejo, S.; Valencia, I.; Michalska, P.; Pajuelo-Lozano, N.; Sanchez-Perez, I.; Leon, R.; Bartha, J.L.; et al. The angiotensin-(1-7)/Mas receptor axis protects from endothelial cell senescence via klotho and Nrf2 activation. Aging Cell 2019, 18, e12913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogi, M. Effect of renin-angiotensin system on senescence. Geriatr. Gerontol. Int. 2020, 20, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.J.; Ding, Y.N.; Pei, X.Y.; Zhao, X.; Hao, L.; Zhang, Z.Q.; Chen, H.Z.; Liu, P. Vascular Transcriptome Profiling Reveals Aging-Related Genes in Angiotensin Ⅱ-Induced Hypertensive Mouse Aortas. Chin. Med. Sci. J. 2020, 35, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef] [Green Version]
- Shan, H.; Bai, X.; Chen, X. Angiotensin II induces endothelial cell senescence via the activation of mitogen-activated protein kinases. Cell Biochem. Funct. 2008, 26, 459–466. [Google Scholar] [CrossRef]
- Li, R.; Mi, X.; Yang, S.; Yang, Y.; Zhang, S.; Hui, R.; Chen, Y.; Zhang, W. Long-term stimulation of angiotensin II induced endothelial senescence and dysfunction. Exp. Gerontol. 2019, 119, 212–220. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef]
- Santilli, F.; D’Ardes, D.; Davì, G. Oxidative stress in chronic vascular disease: From prediction to prevention. Vascul. Pharmacol. 2015, 74, 23–37. [Google Scholar] [CrossRef]
- Suganya, N.; Bhakkiyalakshmi, E.; Sarada, D.V.; Ramkumar, K.M. Reversibility of endothelial dysfunction in diabetes: Role of polyphenols. Br. J. Nutr. 2016, 116, 223–246. [Google Scholar] [CrossRef] [Green Version]
- Huxley, R.; Lee, C.M.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: A systematic review with meta-analysis. Arch. Intern. Med. 2009, 169, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Mostofsky, E.; Rice, M.S.; Levitan, E.B.; Mittleman, M.A. Habitual coffee consumption and risk of heart failure: A dose-response meta-analysis. Circ. Heart Fail. 2012, 5, 401–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravi, F.; Bosetti, C.; Tavani, A.; Bagnardi, V.; Gallus, S.; Negri, E.; Franceschi, S.; La Vecchia, C. Coffee drinking and hepatocellular carcinoma risk: A meta-analysis. Hepatology 2007, 46, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Saito, E.; Inoue, M.; Sawada, N.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Sasazuki, S.; Noda, M.; Iso, H.; Tsugane, S. Association of coffee intake with total and cause-specific mortality in a Japanese population: The Japan Public Health Center-based Prospective Study. Am. J. Clin. Nutr. 2015, 101, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, Y.; Ohie, T.; Yonekawa, Y.; Yonemoto, K.; Aizawa, H.; Mori, Y.; Watanabe, M.; Takeuchi, M.; Hasegawa, M.; Taguchi, C.; et al. Coffee and green tea as a large source of antioxidant polyphenols in the Japanese population. J. Agric. Food Chem. 2009, 57, 1253–1259. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, A.; Currie, J.C.; Desgagnés, J.; Annabi, B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic Acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Lee, J.S.; Jang, H.J.; Kim, S.M.; Chang, M.S.; Park, S.H.; Kim, K.S.; Bae, J.; Park, J.W.; Lee, B.; et al. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur J Pharmacol 2012, 689, 89–95. [Google Scholar] [CrossRef]
- Dhingra, D.; Gahalain, N. Reversal of Reserpine-induced Orofacial Dyskinesia by Chlorogenic Acid in Rats. Pharmacologia 2016, 7, 272–277. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, T.; Itoh, K.; Ruiz, E.; Leake, D.S.; Unoki, H.; Yamamoto, M.; Mann, G.E. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: Activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 2004, 94, 609–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykiotis, G.P.; Bohmann, D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 2008, 14, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging (Albany N. Y.) 2018, 10, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Bellaver, B.; Souza, D.G.; Bobermin, L.D.; Souza, D.O.; Gonçalves, C.A.; Quincozes-Santos, A. Resveratrol Protects Hippocampal Astrocytes Against LPS-Induced Neurotoxicity Through HO-1, p38 and ERK Pathways. Neurochem. Res. 2015, 40, 1600–1608. [Google Scholar] [CrossRef]
- Takano, K.; Tatebe, J.; Washizawa, N.; Morita, T. Curcumin Inhibits Age-Related Vascular Changes in Aged Mice Fed a High-Fat Diet. Nutrients 2018, 10, 1476. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Chen, W.; Gu, X.; Shan, R.; Zou, J.; Liu, G.; Shahid, M.; Gao, J.; Han, B. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget 2017, 8, 14680–14692. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Xiao, C.; Long, F.; Wu, W.; Huang, M.; Qu, L.; Liu, X.; Zhu, Y. Fra-1 plays a critical role in angiotensin II-induced vascular senescence. FASEB J. 2019, 33, 7603–7614. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.L.; Hung, C.H.; Chan, S.H.; Hsieh, P.L.; Ou, H.C.; Cheng, Y.H.; Chu, P.M. Chlorogenic Acid Protects Against oxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, e1700928. [Google Scholar] [CrossRef]
- Li, Y.; Zou, L.; Li, T.; Lai, D.; Wu, Y.; Qin, S. Mogroside V inhibits LPS-induced COX-2 expression/ROS production and overexpression of HO-1 by blocking phosphorylation of AKT1 in RAW264.7 cells. Acta Biochim. Biophys. Sin. (Shanghai) 2019, 51, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Forstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.; Dou, Y.; Li, A. Resveratrol Boosts Cognitive Function by Targeting SIRT1. Neurochem. Res. 2018, 43, 1705–1713. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, W.; Feng, X.; Yang, F.; Qin, H.; Wu, S.; Hou, D.X.; Chen, J. Nrf2⁻ARE Signaling Acts as Master Pathway for the Cellular Antioxidant Activity of Fisetin. Molecules 2019, 24, 708. [Google Scholar] [CrossRef] [Green Version]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Motohashi, H.; Yamamoto, M. The Keap1-Nrf2 system in cancers: Stress response and anabolic metabolism. Front. Oncol. 2012, 2, 200. [Google Scholar] [CrossRef] [Green Version]
- Ndisang, J.F. Synergistic Interaction Between Heme Oxygenase (HO) and Nuclear-Factor E2-Related Factor-2 (Nrf2) against Oxidative Stress in Cardiovascular Related Diseases. Curr.Pharm. Des. 2017, 23, 1465–1470. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Klinge, C.M.; Blankenship, K.A.; Risinger, K.E.; Bhatnagar, S.; Noisin, E.L.; Sumanasekera, W.K.; Zhao, L.; Brey, D.M.; Keynton, R.S. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J. Biol. Chem. 2005, 280, 7460–7468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umebayashi, R.; Uchida, H.A.; Kakio, Y.; Subramanian, V.; Daugherty, A.; Wada, J. Cilostazol Attenuates Angiotensin II-Induced Abdominal Aortic Aneurysms but Not Atherosclerosis in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 903–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuyama, M.; Uchida, H.A.; Hada, Y.; Kakio, Y.; Otaka, N.; Umebayashi, R.; Tanabe, K.; Fujii, Y.; Kasahara, S.; Subramanian, V.; et al. Exogenous Vasohibin-2 Exacerbates Angiotensin II-Induced Ascending Aortic Dilation in Mice. Circ. Rep. 2019, 1, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Uchida, H.A.; Kristo, F.; Rateri, D.L.; Lu, H.; Charnigo, R.; Cassis, L.A.; Daugherty, A. Total lymphocyte deficiency attenuates AngII-induced atherosclerosis in males but not abdominal aortic aneurysms in apoE deficient mice. Atherosclerosis 2010, 211, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Maciag, T.; Hoover, G.A.; Stemerman, M.B.; Weinstein, R. Serial propagation of human endothelial cells in vitro. J. Cell Biol. 1981, 91, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Hirai, K.; Sasahira, T.; Ohmori, H.; Fujii, K.; Kuniyasu, H. Inhibition of heme oxygenase-1 by zinc protoporphyrin IX reduces tumor growth of LL/2 lung cancer in C57BL mice. Int. J. Cancer 2007, 120, 500–505. [Google Scholar] [CrossRef]








| Saline/Saline | Saline/CGA High | Ang II/Saline | AngII/CGA Low | AngII/CGA High | |
|---|---|---|---|---|---|
| N | 4 | 4 | 4 | 4 | 4 |
| BW (g) | 33.5 ± 5.8 | 34.2 ± 5.1 | 29.9 ± 1.4 | 28.0 ± 1.4 | 31.6 ± 3.7 |
| HR (bpm) | 648 ± 28 | 662 ± 7 | 682 ± 43 | 660 ± 86 | 702 ± 23 |
| SBP (mmHg) | 104 ± 7 | 107 ± 9 | 129 ± 5 *,# | 121 ± 13 | 119 ± 7 |
| Saline/Saline | AngII/Saline | AngII/CGA | AngII/CGA/ZnPP | |
|---|---|---|---|---|
| N | 5 | 5 | 5 | 5 |
| BW (g) | 29.1 ± 5.5 | 28.7 ± 1.5 | 29.1 ± 3.8 | 27.2 ± 2.2 |
| HR (bpm) | 643 ± 62 | 663 ± 63 | 629 ± 99 | 564 ± 104 |
| SBP (mmHg) | 109 ± 9 | 127 ± 5 * | 113 ± 9 | 112 ± 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hada, Y.; Uchida, H.A.; Otaka, N.; Onishi, Y.; Okamoto, S.; Nishiwaki, M.; Takemoto, R.; Takeuchi, H.; Wada, J. The Protective Effect of Chlorogenic Acid on Vascular Senescence via the Nrf2/HO-1 Pathway. Int. J. Mol. Sci. 2020, 21, 4527. https://doi.org/10.3390/ijms21124527
Hada Y, Uchida HA, Otaka N, Onishi Y, Okamoto S, Nishiwaki M, Takemoto R, Takeuchi H, Wada J. The Protective Effect of Chlorogenic Acid on Vascular Senescence via the Nrf2/HO-1 Pathway. International Journal of Molecular Sciences. 2020; 21(12):4527. https://doi.org/10.3390/ijms21124527
Chicago/Turabian StyleHada, Yoshiko, Haruhito A. Uchida, Nozomu Otaka, Yasuhiro Onishi, Shugo Okamoto, Mariko Nishiwaki, Rika Takemoto, Hidemi Takeuchi, and Jun Wada. 2020. "The Protective Effect of Chlorogenic Acid on Vascular Senescence via the Nrf2/HO-1 Pathway" International Journal of Molecular Sciences 21, no. 12: 4527. https://doi.org/10.3390/ijms21124527
APA StyleHada, Y., Uchida, H. A., Otaka, N., Onishi, Y., Okamoto, S., Nishiwaki, M., Takemoto, R., Takeuchi, H., & Wada, J. (2020). The Protective Effect of Chlorogenic Acid on Vascular Senescence via the Nrf2/HO-1 Pathway. International Journal of Molecular Sciences, 21(12), 4527. https://doi.org/10.3390/ijms21124527