Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicity of Apigenin in RAW264.7, RBL-2H3, and HaCaT Cells
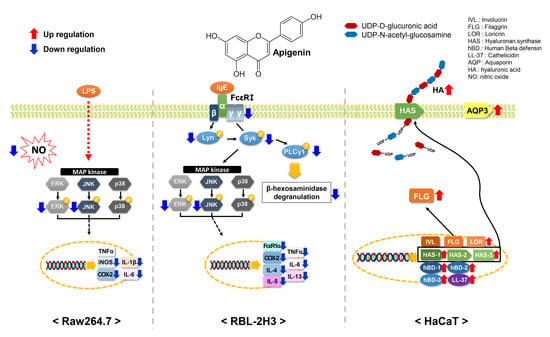
2.2. Effects of Apigenin on NO Production and β-Hexosaminidase Release
2.3. Effects of Apigenin on Cytokines and MAPK Signaling Pathways in RAW264.7 Cells
2.4. Effects of Apigenin on Cytokines, MAPK, and Allergic Signaling Pathways in RBL-2H3 Cells
2.5. Effects of Apigenin on HaCaT Cells
3. Materials and Methods
3.1. Reagents
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. NO and β-Hexosaminidase Release Assay
3.5. Real-Time Quantitative PCR
3.6. Western Blot Analysis
3.7. ELISA
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Chirumbolo, S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition 2017, 33, 311–321. [Google Scholar] [CrossRef]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Kandola, K.; Bowman, A.; Birch-Machin, M.A. Oxidative stress—A key emerging impact factor in health, ageing, lifestyle and aesthetics. Int. J. Cosmet. Sci. 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Milam, E.C.; Rieder, E.A. An approach to cosmeceuticals. J. Drugs Dermatol. 2016, 15, 452–456. [Google Scholar]
- Xie, T.; Song, S.; Li, S.; Ouyang, L.; Xia, L.; Huang, J. Review of natural product databases. Cell Prolif. 2015, 48, 398–404. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Yoon, N.Y. Pathogenesis of atopic dermatitis. J. Korean Med. Assoc. 2014, 57, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.S.; Lee, P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Wang, H.Y.; Jun, M.; Jung, M.; Kim, D.H.; Lee, N.R.; Hong, K.W.; Seo, S.J.; Choi, E.; Lee, J.; et al. Simultaneous detection of barrier-and immune-related gene variations in patients with atopic dermatitis by reverse blot hybridization assay. Clin. Exp. Dermatol. 2018, 43, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Bradding, P.; Walls, A.F.; Holgate, S.T. The role of the mast cell in the pathophysiology of asthma. J. Allergy Clin. Immunol. 2006, 117, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J. New concepts about the mast cell. N. Engl. J. Med. 1993, 328, 257–264. [Google Scholar] [CrossRef]
- Maggi, E. The TH1/TH2 paradigm in allergy. Immunotechnology 1998, 3, 233–244. [Google Scholar] [CrossRef]
- Beaven, M.A.; Metzger, H. Signal transduction by Fc receptors: The Fc Epsilon RI case. Immunol. Today 1993, 14, 222–226. [Google Scholar] [CrossRef]
- Ravetch, J.V.; Kinet, J.P. Fc receptors. Annu. Rev. Immunol. 1991, 9, 457–492. [Google Scholar] [CrossRef]
- Razin, E.; Pecht, I.; Rivera, J. Signal transduction in the activation of mast cells and basophils. Immunol. Today 1995, 16, 370–373. [Google Scholar] [CrossRef]
- Kawakami, Y.; Hartman, S.E.; Holland, P.M.; Cooper, J.A.; Kawakami, T. Multiple signaling pathways for the activation of JNK in mast cells: Involvement of Bruton’s tyrosine kinase, protein kinase C, and JNK kinases, SEK1 and MKK7. J. Immunol. 1998, 161, 1795–1802. [Google Scholar]
- Kawakami, Y.; Miura, T.; Bissonnette, R.; Hata, D.; Khan, W.N.; Kitamura, T.; Maeda-Yamamoto, M.; Hartman, S.E.; Yao, L.; Alt, F.W. Bruton’s tyrosine kinase regulates apoptosis and JNK/SAPK kinase activity. Proc. Natl. Acad. Sci. USA 1997, 94, 3938–3942. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Baumgartner, R.A.; Yamada, K.; Beaven, M.A. Mitogen activated protein (MAP) kinase regulates production of tumor necrosis factor-alpha and release of arachidonic acid in mast cells: Indications of communication between p38 and p42 MAP kinases. J. Biol. Chem. 1997, 272, 13397–13402. [Google Scholar] [CrossRef] [Green Version]
- Karin, M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995, 270, 16483–16486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iontcheva, I.; Amar, S.; Zawawi, K.H.; Kantarci, A.; Van Dyke, T.E. Role for moesin in lipopolysaccharide stimulated signal transduction. Infect. Immun. 2004, 72, 2312–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.Y.; Dawson, V.L.; Dawson, T.M. Neurobiology of nitric oxide. Crit. Rev. Neurobiol. 1996, 10, 291–316. [Google Scholar] [CrossRef]
- Hippeli, S.; Elstner, E.F. Inhibition of biochemical model reactions for inflammatory processes by plant extracts: A review on recent developments. Free Radic. Res. 1999, 31, 81–87. [Google Scholar] [CrossRef]
- McCartney-Francis, N.; Allen, J.B.; Mizel, D.E.; Albina, J.E.; Xie, Q.W.; Nathan, C.F.; Wahl, S.M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J. Exp. Med. 1993, 178, 749–754. [Google Scholar] [CrossRef] [Green Version]
- Masferrer, J.L.; Zweifel, B.S.; Manning, P.T.; Hauser, S.D.; Leahy, K.M.; Smith, W.G.; Isakson, P.C.; Seibert, K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc. Natl. Acad. Sci. USA 1994, 91, 3228–3232. [Google Scholar] [CrossRef] [Green Version]
- Beutler, B.; Cerami, A. The biology of cachectin/TNF-α primary mediator of the host response. Annu. Rev. Immunol. 1989, 7, 625–655. [Google Scholar] [CrossRef]
- Dendorfer, U. Molecular biology of cytokines. Artif. Organs 1996, 20, 437–444. [Google Scholar] [CrossRef]
- Forslin, B. A domain mosaic model of the skin barrier. Acta Derrn. Venereol. 1994, 74, 1–6. [Google Scholar] [CrossRef]
- Nemes, Z.; Steinert, P.M. Brick and mortar of the epidermal barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Steinert, P.M.; Marekov, L.N. The poteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J. Biol. Chem. 1995, 270, 17702–17711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahiya, P.; Kamal, R. Hyaluronic acid: A boon in periodontal therapy. N. Am. J. Med. Sci. 2013, 5, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin water channels from atomic structure to clinical medicine. J. Physiol. 2002, 542, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Sougrat, R.; Morand, M.; Gondran, C.; Barré, P.; Gobin, R.; Bonté, F.; Dumas, M.; Verbavatz, J.M. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J. Investig. Dermatol. 2002, 118, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Song, Y.; Yang, B.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc. Natl. Acad. Sci. USA 2000, 97, 4386–4391. [Google Scholar] [CrossRef] [Green Version]
- Combet, S.; Van Landschoot, M.; Moulin, P.; Piech, A.; Verbavatz, J.M.; Goffin, E.; Balligand, J.L.; Lameire, N.; Devuyst, O. Regulation of aquaporin-1 and nitric oxide synthase isoforms in a rat model of acute peritonitis. J. Am. Soc. Nephrol. 1999, 10, 2185–2196. [Google Scholar]
- Hara, M.; Ma, T.; Verkman, A.S. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J. Biol. Chem. 2002, 277, 46616–46621. [Google Scholar] [CrossRef] [Green Version]
- Hara-Chikuma, M.; Verkman, A.S. Roles of aquaporin-3 in the epidermis. J. Investig. Dermatol. 2008, 128, 2145–2151. [Google Scholar] [CrossRef] [Green Version]
- Braff, M.H.; Di Nardo, A.; Gallo, R.L. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J. Investig. Dermatol. 2005, 124, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Izadpahah, A.; Gallo, R.L. Antimicrobial peptides. J. Am. Acad. Dermatol. 2005, 52, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Leung, D.Y.M. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinholz, M.; Ruzicka, T.; Schauber, J. Cathelicidin LL-37: An antimicrobial peptide with a role in inflammatory skin disease. Ann. Dermatol. 2012, 24, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid-Grendelmeier, P.; Simon, D.; Simon, H.U.; Akdis, C.A.; Wuthrich, B. Epidemiology, clinical features, and immunology of the intrinsic (non-IgE mediated) type of atopic dermatitis (constitutional dermatitis). Allergy 2001, 56, 841–849. [Google Scholar] [CrossRef]
- Wollenberg, A.; Bieber, T. Atopic dermatitis: From the genes to skin lesions. Allergy 2000, 55, 205–213. [Google Scholar] [CrossRef]
- Avena-Woods, C. Overview of atopic dermatitis. Am. J. Magna Care 2017, 23, S115–S123. [Google Scholar]
- Park, C.H.; Park, J.H.; Min, S.Y.; Kim, K.; Kim, S.; Park, Y.J. Studies on antioxidant, anti-inflammation and whitening activities of Hordeum vulgare L. extracts and their fractions. J. Soc. Cosmet. Sci. Korea 2019, 45, 287–297. [Google Scholar] [CrossRef]
- Eun, C.S.; Hwang, E.Y.; Lee, S.O.; Yang, S.A.; Yu, M.H. Anti-oxidant and anti-inflammatory activities of barley sprout extract. J. Life Sci. 2016, 26, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.H.; Cho, C.Y.; Ha, K.S.; Choi, E.J.; Kang, Y.R.; Kwon, Y.I. The antioxidant and antimicrobial activities of extracts of selected barley and wheat inhabited in Korean Peninsula. J. Korean Soc. Food. Sci. Nutr. 2013, 42, 1003–1007. [Google Scholar] [CrossRef] [Green Version]
- Nirupama, G.; Mohammad, B.H.; Dilip, K.R.; Nigel, P.B. A Review of extraction and analysis of bioactives in oat and barley and scope for use of novel food processing technologies. Molecules 2015, 20, 10884–10909. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.S.; Hoe, Y.H.; Moon, E.Y.; Kan, M.H. Physiology activity of barley leaf using different drying methods. J. Korean Soc. Food. Sci. Nutr. 2008, 37, 1627–1631. [Google Scholar] [CrossRef]
- Yang, Y.K.; Kim, J.Y.; Kwon, O. Development of flavonoid database for commonly consumed foods by Koreans. Korean J. Nutr. 2012, 45, 283–292. [Google Scholar] [CrossRef]
- The Flavonoid Database 1.0. Available online: http://koreanfood.rda.go.kr/kfi/fct/fctCompSrch/list# (accessed on 6 May 2020).
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef]
- Holden, J.M.; Bhagwat, S.A.; Haytowitz, D.B.; Gebhardt, S.E.; Dwyer, J.T.; Peterson, J.; Beecher, G.R.; Eldridge, A.L.; Balentine, D. Development of a database of critically evaluated flavonoids data: Application of USDA’s data quality evaluation system. J. Food Compost. Anal. 2005, 18, 829–844. [Google Scholar] [CrossRef]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef]
- Dickancaité, E.; Nemeikaité, A.; Kalvelytè, A.; Cènas, N. Prooxidant character of flavonoid cytotoxicity: Structure-activity relationships. Biochem. Mol. Biol. Int. 1998, 45, 923–930. [Google Scholar] [CrossRef]
- Sahu, S.C.; Gray, G.C. Lipid peroxidation and DNA damage induced by morin and naringenin in isolatedrat liver nuclei. Food Chem. Toxicol. 1997, 35, 443–447. [Google Scholar] [CrossRef]
- Hilliquin, P.; Borderie, D.; Hernvann, A.; Menkes, C.J.; Ekindjian, O.G. Nitric oxide as S-nitrosoproteins in rheumatoid arthritis. Arthritis Rheum. 1997, 40, 1512–1517. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Asp. Med. 2010, 31, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Fukuishi, N.; Murakami, S.; Ohno, A.; Yamanaka, N.; Matsui, N.; Fukutsuji, K.; Yamada, S.; Itoh, K.; Akagi, M. Does β-hexosaminidase function only as a degranulation indicator in mast cells? The primary role of β-hexosaminidase in mast cell granules. J. Immunol. 2014, 193, 1886–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laupacis, A.; Keown, P.A.; Ulan, R.A.; McKenzie, N.; Stiller, C.R. Cyclosporin A: A powerful immunosuppressant. Can. Med. Assoc. J. 1982, 126, 1041–1046. [Google Scholar]
- Walsh, L.J.; Trinchieri, G.; Waldorf, H.A.; Whitaker, D.; Murphy, G.F. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule. Proc. Natl. Acad. Sci. USA 1991, 88, 4220–4224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, K.; Torres, R. Role of interleukin-1beta During Pain and Inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Jean, Y.H.; Chen, W.F.; Duh, C.Y.; Huang, S.Y.; Hsu, C.H.; Lin, C.S.; Sung, C.S.; Chen, I.M.; Wen, Z.H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S.; et al. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 2018, 41, 888–898. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- Hidding, U.; Mielke, K.; Waetzig, V.; Brecht, S.; Hanisch, U.; Behrens, A.; Wagner, E.; Herdegen, T. The c-Jun N-terminal kinases in cerebral microglia: Immunological functions in the brain. Biochem. Pharmacol. 2002, 64, 781–788. [Google Scholar] [CrossRef]
- Waetzig, V.; Czeloth, K.; Hidding, U.; Mielke, K.; Kanzow, M.; Brecht, S.; Goetz, M.; Lucius, R.; Herdegen, T.; Hanisch, U.K. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 2005, 50, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacGlashan, D., Jr. IgE receptor and signal transduction in mast cells and basophils. Curr. Opin. Immunol. 2008, 20, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Dienz, O.; Rincon, M. The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 2009, 130, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, D.D. Mast cells and mastocytosis. Blood 2009, 112, 946–956. [Google Scholar] [CrossRef] [Green Version]
- Neveu, W.A.; Allard, J.B.; Dienz, O.; Wargo, M.J.; Ciliberto, G.; Whittaker, L.A.; Rincon, M. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J. Immunol. 2009, 183, 1732–1738. [Google Scholar] [CrossRef]
- Song, Z.; Casolaro, V.; Chen, R.; Georas, S.N.; Monos, D.; Ono, S.J. Polymorphic nucleotides within the human IL-4 promoter that mediate overexpression of the gene. J. Immunol. 1996, 156, 424–429. [Google Scholar] [PubMed]
- Fish, S.C.; Donaldson, D.D.; Goldman, S.J.; Williams, C.M.M.; Kasaian, M.T. IgE generation and mast cell effector function in mice deficient in IL-4 and IL-13. J. Immunol. 2005, 174, 7716–7724. [Google Scholar] [CrossRef] [Green Version]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef] [Green Version]
- Soumelis, V.; Liu, Y.J. Human thymic stromal lymphopoietin: A novel epithelial cell-derived cytokine and a potential key player in the induction of allergic inflammation. Springer Semin. Immunopathol. 2004, 25, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Van Joost, T.; Kozel, M.M.; Tank, B.; Troost, R.; Prrens, E.P. Cyclosporine in atopic dermatitis: Modulation in the expression of immunologic markers in lesional skin. J. Am. Acad. Dermatol. 1992, 27, 922–928. [Google Scholar] [CrossRef]
- Baugh, J.A.; Bucala, R. Mechanisms for modulating TNF alpha in immune and inflammatory disease. Curr. Opin. Drug Discov. Dev. 2001, 4, 635–650. [Google Scholar]
- Meyer, O. Role of TNF-alpha and cytokines in the physiopathology of rheumatoid arthritis. Therapeutic perspectives. Bull. Acad. Natl. Med. 2003, 187, 935–954. [Google Scholar]
- Weiss, E.; Mamelak, J.A.; La Morgia, S.; Wang, B.; Feliciani, C.; Tulli, A.; Sauder, D.N. The role of interleukin 10 in the pathogenesis andpotential treatment of skin diseases. J. Am. Acad. Dermatol. 2004, 50, 657–675. [Google Scholar] [CrossRef]
- Paolini, R.; Jouvin, M.H.; Kinet, J.P. Phosphorylation and dephosphorylation of the high-affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature 1991, 353, 855–858. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef]
- Rivera, J.; Gilfillan, A.M. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 2006, 117, 1214–1225. [Google Scholar] [CrossRef]
- Theoharidies, T.C.; Kalogeromitros, D. The critical role of mast cells in allergy and inflammation. Ann. N. Y. Acad. Sci. 2006, 1088, 78–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, R.L.; Murakami, M.; Ohtake, T.; Zaiou, M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J. Allergy Clin. Immunol. 2002, 110, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.J.; Irvine, A.D.; Terron-Kwiatkowski, A.; Sandilands, A.; Campbell, L.E.; Zhao, Y.; Liao, H.; Evans, A.T.; Goudie, D.R.; Lewis-Jones, S.; et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat. Genet. 2006, 38, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Clausen, M.L.; Agner, T. Antimicrobial peptides, infections and the skin barrier. Curr. Probl. Dermatol. 2016, 49, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Roudier, N.; Bailly, P.; Gane, P.; Lucien, N.; Gobin, R.; Cartron, J.P.; Ripoche, P. Erythroid expression and oligomeric state of the AQP3 protein. J. Biol. Chem. 2002, 277, 7664–7669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, Y.; Ota, Y.; Hara, M.; Inoue, S. Osmotic stress up-regulates aquaporin-3 gene expression in cultured human keratinocytes. Biochim. Biophys. Acta 2001, 1522, 82–88. [Google Scholar] [CrossRef]
- Brown, M.B.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef]
- Ghersetich, I.; Notti, T.; Gampainle, G.; Grappone, C.; Dini, G. Hyalurinic acid in cutaneous intrinsic aging. Int. J. Dermatol. 1994, 33, 119–122. [Google Scholar] [CrossRef]
- Longas, M.O.; Russell, C.S.; He, X.Y. Evidence for structural changes in dermatan sulfate and hyaluronic acid with aging. Carbohydr. Res. 1987, 159, 127–136. [Google Scholar] [CrossRef]
- Malaisse, J.; Bourguignon, V.; De Vuyst, E.; de Rouvroit, C.L.; Nikkels, A.F.; Flamion, B.; Poumay, Y. Hyaluronan metabolism in human keratinocytes and atopic dermatitis skin is driven by a balance of hyaluronan synthases 1 and 3. J. Investig. Dermatol. 2014, 134, 2174–2182. [Google Scholar] [CrossRef] [Green Version]
- Pienimäki, J.P.; Rilla, K.; Fülöp, C.; Sironen, R.K.; Karvinen, S.; Pasonen, S.; Lammi, M.J.; Tammi, R.; Hascall, V.C.; Tammi, M.I. Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J. Biol. Chem. 2001, 276, 20428–20435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, R.S.; Falconer, A.; Ikram, M.; Bissett, C.E.; Cerio, R.; Quinn, A.G. Expression of the peptide antibiotics human beta defensin-1 and human beta defensin-2 in normal human skin. J. Investig. Dermatol. 2001, 117, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, T. Defensins and host defense. Science 1999, 286, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Bals, R. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 2000, 1, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Braff, M.H.; Bardan, A.; Nizet, V.; Gallo, R.L. Cutaneous defense mechanisms by antimicrobial peptides. J. Investig. Dermatol. 2005, 125, 9–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, J.M.; Harder, J. Antimicrobial skin peptides and proteins. Cell. Mol. Life Sci. 2006, 63, 469–486. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-H.; Min, S.-Y.; Yu, H.-W.; Kim, K.; Kim, S.; Lee, H.-J.; Kim, J.-H.; Park, Y.-J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. https://doi.org/10.3390/ijms21134620
Park C-H, Min S-Y, Yu H-W, Kim K, Kim S, Lee H-J, Kim J-H, Park Y-J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. International Journal of Molecular Sciences. 2020; 21(13):4620. https://doi.org/10.3390/ijms21134620
Chicago/Turabian StylePark, Che-Hwon, Seon-Young Min, Hye-Won Yu, Kyungmin Kim, Suyeong Kim, Hye-Ja Lee, Ji-Hye Kim, and Young-Jin Park. 2020. "Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities" International Journal of Molecular Sciences 21, no. 13: 4620. https://doi.org/10.3390/ijms21134620
APA StylePark, C. -H., Min, S. -Y., Yu, H. -W., Kim, K., Kim, S., Lee, H. -J., Kim, J. -H., & Park, Y. -J. (2020). Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. International Journal of Molecular Sciences, 21(13), 4620. https://doi.org/10.3390/ijms21134620