Indonesian Ginger (Bangle) Extract Promotes Neurogenesis of Human Neural Stem Cells through WNT Pathway Activation
Abstract
:1. Introduction
2. Results
2.1. Bangle (Zingiber purpureum) Extract Promotes Neuronal Differentiation of hfNSCs and Enhanced Neurite Outgrowth of Immature Neurons
2.2. Bangle Extract Affects the Expression of Genes Related to Neurogenesis and WNT Pathway
2.3. Bangle Extract Activates the Canonical WNT/β-Catenin Signaling Pathway in hfNSCs
2.4. Canonical WNT/β-Catenin Signaling Is Responsible for the Promotion of Neuronal Differentiation in Response to Bangle Extract Treatment
2.5. Bangle Extract Affects Histone Modifications during Neuronal Differentiation
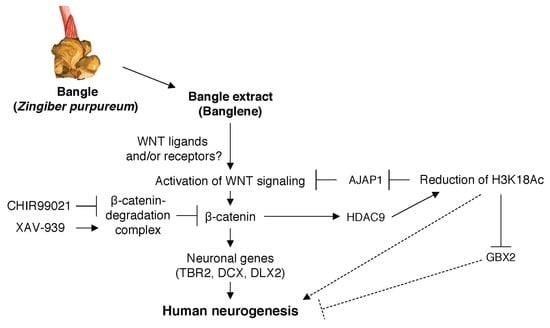
3. Discussion
4. Material and Methods
4.1. Human Fetal Neural Stem Cell Culture, In Vitro Differentiation and Immunostaining
4.2. Bangle Extract and cis/trans-BANGLENE
4.3. Measurement of Neurite Length
4.4. Analysis of Nuclear Translocation of β-Catenin by Immunocytochemistry
4.5. Quantitative Reverse-Transcription Polymerase Chain Reaction
4.6. Nuclear Extract and Western Blot Analysis
4.7. Microarray and Gene Ontology Analysis
4.8. Histone H3 Modification Analysis by ELISA
4.9. Chromatin-Immunoprecipitation Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NSCs | Neural stem cells |
| SAMP8 | Senescence Accelerated Mouse-Prone 8 |
| APP | amyloid precursor protein |
| PI | propidium iodide |
| EdU | 5-ethynyl-2′-deoxyuridine |
| DMSO | dissolved in dimethyl sulfoxide |
| DCX | doublecortin |
| TBR2 | T-box brain protein 2 |
| DLX2 | Distal-less homeobox 2 |
| AJAP1 | adherens junctions associated arotein 1 |
| GBX2 | gastrulation brain homeobox 2 |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| H3K18Ac | acetylated histone H3 lysine 18 |
| HDAC9 | histone deacetylase-9 |
| ChIP | chromatin immunoprecipitation |
| NPCs | neuronal progenitor cells |
| GSK3β | glycogen synthase kinase 3β |
| LSD1 | lysine specific demethylase 1 |
| FAD | flavin adenine dinucleotide |
| GADD45G | Growth Arrest And DNA Damage Inducible Gamma |
References
- Fujita, S. The discovery of the matrix cell, the identification of the multipotent neural stem cell and the development of the central nervous system. Cell Struct. Funct. 2003, 28, 205–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noctor, S.C.; Flint, A.C.; Weissman, T.A.; Dammerman, R.S.; Kriegstein, A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 2001, 409, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Takouda, J.; Katada, S.; Nakashima, K. Emerging mechanisms underlying astrogenesis in the developing mammalian brain. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 386–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adefuin, A.M.; Kimura, A.; Noguchi, H.; Nakashima, K.; Namihira, M. Epigenetic mechanisms regulating differentiation of neural stem/precursor cells. Epigenomics 2014, 6, 637–649. [Google Scholar] [CrossRef]
- Roidl, D.; Hacker, C. Histone methylation during neural development. Cell Tissue Res. 2014, 356, 539–552. [Google Scholar] [CrossRef]
- Borrell, V.; Reillo, I. Emerging roles of neural stem cells in cerebral cortex development and evolution. Dev. Neurobiol. 2012, 72, 955–971. [Google Scholar] [CrossRef]
- Hirano, K.; Namihira, M. New insight into LSD1 function in human cortical neurogenesis. Neurogenesis (Austin) 2016, 3, e1249195. [Google Scholar] [CrossRef] [Green Version]
- Letinic, K.; Zoncu, R.; Rakic, P. Origin of GABAergic neurons in the human neocortex. Nature 2002, 417, 645–649. [Google Scholar] [CrossRef]
- Kaewchoothong, A.; Tewtrakul, S.; Panichayupakaranant, P. Inhibitory Effect of Phenylbutanoid-rich Zingiber cassumunar Extracts on Nitric Oxide Production by Murine Macrophage-like RAW264.7 Cells. Phytother. Res. 2012, 26, 1789–1792. [Google Scholar] [CrossRef]
- Nakamura, S.; Iwami, J.; Matsuda, H.; Wakayama, H.; Pongpiriyadacha, Y.; Yoshikawa, M. Structures of New Phenylbutanoids and Nitric Oxide Production Inhibitors from the Rhizomes of Zingiber cassumunar. Chem. Pharm. Bull. 2009, 57, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, Y.; Kawahara, N.; Harada, M. Anti-inflammatory effect of Zingiber cassumunar Roxb. and its active principles. Chem. Pharm. Bull. (Tokyo) 1991, 39, 2353–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suksaeree, J.; Charoenchai, L.; Madaka, F.; Monton, C.; Sakunpak, A.; Charoonratana, T.; Pichayakorn, W. Zingiber cassumunar blended patches for skin application: Formulation, physicochemical properties, and in vitro studies. Asian J. Pharm. Sci. 2015, 10, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Suksaeree, J.; Monton, C.; Charoenchai, L.; Madaka, F.; Chusut, T. Determination of (E)-4-(3′, 4′-dimethoxyphenyl)but-3-en-1-ol content in Zingiber cassumunar ROXB. (Plai) patches. Int. J. Pharma Pharma Sci. 2014, 6, 434–436. [Google Scholar]
- Anasamy, T.; Abdul, A.B.; Sukari, M.A.; Abdelwahab, S.I.; Mohan, S.; Kamalidehghan, B.; Azid, M.Z.; Nadzri, N.M.; Andas, A.R.J.; Beng, N.K.; et al. A Phenylbutenoid Dimer, cis-3-(3′,4′-Dimethoxyphenyl)-4-[(E)-3′′′, 4′′′-Dimethoxystyryl]Cyclohex-1-ene, Exhibits Apoptogenic Properties in T-Acute Lymphoblastic Leukemia Cells via Induction of p53-Independent Mitochondrial Signalling Pathway. Evid-Based Compl. Alt. 2013, 2013, 939810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limvuttegrijerat, T.; Poachanukoon, O.; Koontongkaew, S.; Ayudhya, T.D.N. Crude ethanolic extracts of Zingiber cassumunar ROXB. inhibit PMA-induced MUC2 and MUC5AC expression via ERK inhibition in human airway epithelial cells. Asian Pac. J. Allergy 2014, 32, 328–336. [Google Scholar]
- Park, J.; Chung, H.; Bang, S.H.; Han, A.R.; Seo, E.K.; Chang, S.E.; Kang, D.H.; Oh, E.S. (E)-4-(3,4-Dimethoxyphenyl)but-3-en-1-ol Enhances Melanogenesis through Increasing Upstream Stimulating Factor-1-Mediated Tyrosinase Expression. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Poachanukoon, O.; Meesuk, L.; Pattanacharoenchai, N.; Monthanapisut, P.; Dechatiwongse Na Ayudhya, T.; Koontongkaew, S. Zingiber cassumunar ROXb. and its active constituent inhibit MMP-9 direct activation by house dust mite allergens and MMP-9 expression in PMA-stimulated human airway epithelial cells. Asian Pac. J. Allergy Immunol. 2015, 33, 42–51. [Google Scholar] [CrossRef]
- Matsui, N.; Kido, Y.; Okada, H.; Kubo, M.; Nakai, M.; Fukuishi, N.; Fukuyama, Y.; Akagi, M. Phenylbutenoid dimers isolated from Zingiber purpureum exert neurotrophic effects on cultured neurons and enhance hippocampal neurogenesis in olfactory bulbectomized mice. Neurosci. Lett. 2012, 513, 72–77. [Google Scholar] [CrossRef]
- Nakai, M.; Iizuka, M.; Matsui, N.; Hosogi, K.; Imai, A.; Abe, N.; Shiraishi, H.; Hirata, A.; Yagi, Y.; Jobu, K.; et al. Bangle (Zingiber purpureum) Improves Spatial Learning, Reduces Deficits in Memory, and Promotes Neurogenesis in the Dentate Gyrus of Senescence-Accelerated Mouse P8. J. Med. Food 2016, 19, 435–441. [Google Scholar] [CrossRef]
- Kubo, M.; Gima, M.; Baba, K.; Nakai, M.; Harada, K.; Suenaga, M.; Matsunaga, Y.; Kato, E.; Hosoda, S.; Fukuyama, Y. Novel neurotrophic phenylbutenoids from Indonesian ginger Bangle, Zingiber purpureum. Bioorg. Med. Chem. Lett. 2015, 25, 1586–1591. [Google Scholar] [CrossRef]
- Tan, X.; Apte, U.; Micsenyi, A.; Kotsagrelos, E.; Luo, J.H.; Ranganathan, S.; Monga, D.K.; Bell, A.; Michalopoulos, G.K.; Monga, S.P. Epidermal growth factor receptor: A novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology 2005, 129, 285–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jho, E.H.; Zhang, T.; Domon, C.; Joo, C.K.; Freund, J.N.; Costantini, F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002, 22, 1172–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirabayashi, Y.; Itoh, Y.; Tabata, H.; Nakajima, K.; Akiyama, T.; Masuyama, N.; Gotoh, Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development 2004, 131, 2791–2801. [Google Scholar] [CrossRef] [Green Version]
- Millet, S.; Campbell, K.; Epstein, D.J.; Losos, K.; Harris, E.; Joyner, A.L. A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature 1999, 401, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, F.; Xiang, G.; Cao, L.; Wang, S.; Liu, J.; Meng, Q.; Xu, D.; Lv, S.; Jiao, J.; et al. beta-Catenin nuclear localization positively feeds back on EGF/EGFR-attenuated AJAP1 expression in breast cancer. J. Exp. Clin. Cancer Res. 2019, 38, 238. [Google Scholar] [CrossRef] [Green Version]
- Hirano, K.; (AIST, Tsukuba, Ibaraki, Japan); Namihira, M.; (AIST, Tsukuba, Ibaraki, Japan). Personal communication, 2020.
- Fullgrabe, J.; Kavanagh, E.; Joseph, B. Histone onco-modifications. Oncogene 2011, 30, 3391–3403. [Google Scholar] [CrossRef] [Green Version]
- Oki, S.; Ohta, T.; Shioi, G.; Hatanaka, H.; Ogasawara, O.; Okuda, Y.; Kawaji, H.; Nakaki, R.; Sese, J.; Meno, C. ChIP-Atlas: A data-mining suite powered by full integration of public ChIP-seq data. EMBO Rep. 2018, 19. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, C.; Sharma, M.; Henderson, B.R. Regulation of beta-catenin nuclear dynamics by GSK-3beta involves a LEF-1 positive feedback loop. Traffic 2011, 12, 983–999. [Google Scholar] [CrossRef]
- Krieghoff, E.; Behrens, J.; Mayr, B. Nucleo-cytoplasmic distribution of beta-catenin is regulated by retention. J. Cell Sci. 2006, 119, 1453–1463. [Google Scholar] [CrossRef] [Green Version]
- Cadigan, K.M.; Waterman, M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Englund, C.; Fink, A.; Lau, C.; Pham, D.; Daza, R.A.; Bulfone, A.; Kowalczyk, T.; Hevner, R.F. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 2005, 25, 247–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sessa, A.; Mao, C.A.; Hadjantonakis, A.K.; Klein, W.H.; Broccoli, V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron 2008, 60, 56–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengoa-Vergniory, N.; Gorrono-Etxebarria, I.; Lopez-Sanchez, I.; Marra, M.; Di Chiaro, P.; Kypta, R. Identification of Noncanonical Wnt Receptors Required for Wnt-3a-Induced Early Differentiation of Human Neural Stem Cells. Mol. Neurobiol. 2017, 54, 6213–6224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanth, P.; Han, K.; Callahan, D.G.; Makovkina, E.; Muratore, C.R.; Lalli, M.A.; Zhou, H.; Boyd, J.D.; Kosik, K.S.; Selkoe, D.J.; et al. Genomic DISC1 Disruption in hiPSCs Alters Wnt Signaling and Neural Cell Fate. Cell Rep. 2015, 12, 1414–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backman, M.; Machon, O.; Mygland, L.; van den Bout, C.J.; Zhong, W.; Taketo, M.M.; Krauss, S. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev. Biol. 2005, 279, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Braun, M.M.; Etheridge, A.; Bernard, A.; Robertson, C.P.; Roelink, H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development 2003, 130, 5579–5587. [Google Scholar] [CrossRef] [Green Version]
- Le, T.N.; Zhou, Q.P.; Cobos, I.; Zhang, S.; Zagozewski, J.; Japoni, S.; Vriend, J.; Parkinson, T.; Du, G.; Rubenstein, J.L.; et al. GABAergic Interneuron Differentiation in the Basal Forebrain Is Mediated through Direct Regulation of Glutamic Acid Decarboxylase Isoforms by Dlx Homeobox Transcription Factors. J. Neurosci. 2017, 37, 8816–8829. [Google Scholar] [CrossRef] [Green Version]
- Pinnock, S.B.; Blake, A.M.; Platt, N.J.; Herbert, J. The roles of BDNF, pCREB and Wnt3a in the latent period preceding activation of progenitor cell mitosis in the adult dentate gyrus by fluoxetine. PLoS ONE 2010, 5, e13652. [Google Scholar] [CrossRef] [Green Version]
- Mendez-David, I.; Guilloux, J.P.; Papp, M.; Tritschler, L.; Mocaer, E.; Gardier, A.M.; Bretin, S.; David, D.J. S 47445 Produces Antidepressant- and Anxiolytic-Like Effects through Neurogenesis Dependent and Independent Mechanisms. Front. Pharmacol. 2017, 8, 462. [Google Scholar] [CrossRef] [Green Version]
- Hirano, K.; Namihira, M. FAD influx enhances neuronal differentiation of human neural stem cells by facilitating nuclear localization of LSD1. FEBS Open Bio 2017, 7, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Cao, Q.; Reilly, C.M.; Young, N.L.; Garcia, B.A.; Mishra, N. Histone deacetylase 9 deficiency protects against effector T cell-mediated systemic autoimmunity. J. Biol. Chem. 2011, 286, 28833–28843. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Chen, H.; Yin, M.; Ye, X.; Chen, G.; Zhou, X.; Yin, L.; Zhang, C.; Ding, B. MiR-376a and histone deacetylation 9 form a regulatory circuitry in hepatocellular carcinoma. Cell Physiol. Biochem. 2015, 35, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.; Alrahbeni, T.M.; Clair, D.S.; Blackwood, D.H.; International Schizophrenia, C.; McCaig, C.D.; Shen, S. HDAC9 is implicated in schizophrenia and expressed specifically in post-mitotic neurons but not in adult neural stem cells. Am. J. Stem Cells 2012, 1, 31–41. [Google Scholar]
- Mejat, A.; Ramond, F.; Bassel-Duby, R.; Khochbin, S.; Olson, E.N.; Schaeffer, L. Histone deacetylase 9 couples neuronal activity to muscle chromatin acetylation and gene expression. Nat. Neurosci. 2005, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sugo, N.; Oshiro, H.; Takemura, M.; Kobayashi, T.; Kohno, Y.; Uesaka, N.; Song, W.J.; Yamamoto, N. Nucleocytoplasmic translocation of HDAC9 regulates gene expression and dendritic growth in developing cortical neurons. Eur. J. Neurosci. 2010, 31, 1521–1532. [Google Scholar] [CrossRef]
- Barreto, G.; Schafer, A.; Marhold, J.; Stach, D.; Swaminathan, S.K.; Handa, V.; Doderlein, G.; Maltry, N.; Wu, W.; Lyko, F.; et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 2007, 445, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C.; Schafer, A. Active DNA demethylation by Gadd45 and DNA repair. Trends Cell Biol. 2012, 22, 220–227. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Pasca, S.P. The rise of three-dimensional human brain cultures. Nature 2018, 553, 437–445. [Google Scholar] [CrossRef]
- Seto, Y.; Eiraku, M. Human brain development and its in vitro recapitulation. Neurosci. Res. 2019, 138, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Kubo, M.; Okamoto, Y.; Matsunaga, Y.; Kyo, H.; Suzuki, N.; Uebaba, K.; Fukuyama, Y. Safety Assessment of Bangle (Zingiber purpureum Rosc.) Rhizome Extract: Acute and Chronic Studies in Rats and Clinical Studies in Human. ACS Omega 2018, 3, 15879–15889. [Google Scholar] [CrossRef] [PubMed]
- Taub, E.; Uswatte, G.; Elbert, T. New treatments in neurorehabilitation founded on basic research. Nat. Rev. Neurosci. 2002, 3, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippert, T.; Watson, N.; Ji, X.; Yasuhara, T.; Date, I.; Kaneko, Y.; Tajiri, N.; Borlongan, C.V. Detrimental effects of physical inactivity on neurogenesis. Brain Circ. 2016, 2, 80–85. [Google Scholar] [CrossRef]
- Yasuhara, T.; Kameda, M.; Sasaki, T.; Tajiri, N.; Date, I. Cell Therapy for Parkinson’s Disease. Cell Transplant. 2017, 26, 1551–1559. [Google Scholar] [CrossRef]
- Hirano, K.; Namihira, M. LSD1 Mediates Neuronal Differentiation of Human Fetal Neural Stem Cells by Controlling the Expression of a Novel Target Gene, HEYL. Stem. Cells 2016, 34, 1872–1882. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Kataoka, S.; Sato, M.; Takahashi, A.; Norikura, T.; Mukai, Y. Effect of Bangle (Zingiber purpureum) extract and low-intensity exercise on mTOR phosphorylation and autophagy flux in skeletal muscles of rats on a high-fat diet. J. Funct. Foods 2018, 47, 554–561. [Google Scholar] [CrossRef]
- Thomas, P.D.; Kejariwal, A.; Campbell, M.J.; Mi, H.; Diemer, K.; Guo, N.; Ladunga, I.; Ulitsky-Lazareva, B.; Muruganujan, A.; Rabkin, S.; et al. PANTHER: A browsable database of gene products organized by biological function, using curated protein family and subfamily classification. Nucleic Acids Res. 2003, 31, 334–341. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirano, K.; Kubo, M.; Fukuyama, Y.; Namihira, M. Indonesian Ginger (Bangle) Extract Promotes Neurogenesis of Human Neural Stem Cells through WNT Pathway Activation. Int. J. Mol. Sci. 2020, 21, 4772. https://doi.org/10.3390/ijms21134772
Hirano K, Kubo M, Fukuyama Y, Namihira M. Indonesian Ginger (Bangle) Extract Promotes Neurogenesis of Human Neural Stem Cells through WNT Pathway Activation. International Journal of Molecular Sciences. 2020; 21(13):4772. https://doi.org/10.3390/ijms21134772
Chicago/Turabian StyleHirano, Kazumi, Miwa Kubo, Yoshiyasu Fukuyama, and Masakazu Namihira. 2020. "Indonesian Ginger (Bangle) Extract Promotes Neurogenesis of Human Neural Stem Cells through WNT Pathway Activation" International Journal of Molecular Sciences 21, no. 13: 4772. https://doi.org/10.3390/ijms21134772
APA StyleHirano, K., Kubo, M., Fukuyama, Y., & Namihira, M. (2020). Indonesian Ginger (Bangle) Extract Promotes Neurogenesis of Human Neural Stem Cells through WNT Pathway Activation. International Journal of Molecular Sciences, 21(13), 4772. https://doi.org/10.3390/ijms21134772