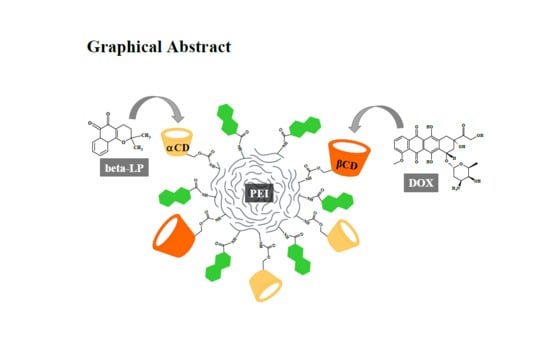
Controlled Drug Release and Cytotoxicity Studies of Beta-Lapachone and Doxorubicin Loaded into Cyclodextrins Attached to a Polyethyleneimine Matrix
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Polymeric Drug Carrier
2.2. Selective Loading of Beta-LP and DOX into PEI-βCD-αCD-FA Nanoconjugate
2.3. Size and Stability of PEI-βCD(DOX)-αCD(beta-LP)-FA Nanoconjugate
2.4. Degradation Pathway of PEI-βCD(DOX)-αCD(beta-LP)-FA Nanoconjugate
2.5. Biological Screening
3. Materials and Methods
3.1. Materials
3.2. Synthesis of PEI-βCD-αCD-FA Nanoconjugate and its Complex with DOX and Beta-LP
3.3. Synthesis of PEI-βCD
3.4. Synthesis of PEI-βCD-αCD
3.5. Synthesis of PEI-βCD-αCD-FA
3.6. Cell Culture
3.7. Biological Screening
3.8. Applied Characterization Techniques
3.9. Kinetic Analysis of Drug Release In Vitro Profiles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| beta-LP | beta-lapachone |
| CD | cyclodextrins |
| DLS | dynamic light scattering |
| DOX | doxorubicin |
| FA | folic acid |
| FR | folate receptor |
| FTIR | Fourier-transform infrared spectroscopy |
| IC50 | half maximal inhibitory concentration |
| NMR | nuclear magnetic resonance spectroscopy |
| PBS | phosphate-buffered saline |
| PEI | polyethylenimine |
| PEI-αCD | nanoconjugate of polyethylenimine with alpfa cyclodextrins |
| PEI-βCD | nanoconjugate of polyethylenimine with beta cyclodextrins |
| PEI-βCD-αCD-FA | nanoconjugate of polyethylenimine with alpha and beta cyclodextrins and folic acid |
| PEI-βCD(DOX)-αCD(beta-LP)-FA | nanoconjugate of polyethylenimine with alpha and beta cyclodextrins and folic acid loaded with doxorubicin and beta-lapachone |
| QCM-D | quartz crystal microbalance with dissipation monitoring |
| ZP | zeta potential |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divekar, K.; Swamy, S.; Murugan, V. Synthesis and evaluation of some newer pyrazolines as possible potential antitumor agents. Int. J. Pharm. Sci. Res. 2020, 12, 671–677. [Google Scholar]
- Kuo, Y.-S.; Zheng, M.-Y.; Huang, M.-F.; Miao, C.-C.; Yang, L.-H.; Huang, T.-W.; Chou, Y.-T. Association of Divergent Carcinoembryonic Antigen Patterns and Lung Cancer Progression. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Leal, A.I.C.; Van Grieken, N.C.T.; Palsgrove, D.N.; Phallen, J.; Medina, J.E.; Hruban, C.; Broeckaert, M.A.M.; Anagnostou, V.; Adleff, V.; Bruhm, D.C.; et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, Y.; Sun, L.; Gu, Y.; Hu, L. Synthesis and structure-activity relationship study of water-soluble carbazole sulfonamide derivatives as new anticancer agents. Eur. J. Med. Chem. 2020, 191, 112181. [Google Scholar] [CrossRef] [PubMed]
- Reyna, M.A.; Haan, D.; Paczkowska, M.; Verbeke, L.P.C.; Vazquez, M.; Kahraman, A.; Pulido-Tamayo, S.; Barenboim, J.; Wadi, L.; Dhingra, P.; et al. Pathway and network analysis of more than 2500 whole cancer genomes. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Saraiva, N.; Costa, J.G.; Reis, C.P.; Almeida, N.; Rijo, P.; Fernandes, A.S. Anti-Migratory and Pro-Apoptotic Properties of Parvifloron D on Triple-Negative Breast Cancer Cells. Biomolecules 2020, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wang, D.; Huang, J.; Hu, Y.; Xu, Y. Application of capsaicin as a potential new therapeutic drug in human cancers. J. Clin. Pharm. Ther. 2019, 45, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Brunton, L.L.; Lazo, J.S.; Parker, K.; O Buxton, I.L.; Blumenthal, D.; Csákó, G. Book Review: Goodman and Gilman’s The Pharmacological Basis of Therapeutics: Digital Edition, 11th Edition. Ann. Pharmacother. 2006, 40, 1218. [Google Scholar] [CrossRef]
- Fortuni, B.; Inose, T.; Ricci, M.; Fujita, Y.; Van Zundert, I.; Masuhara, A.; Fron, E.; Mizuno, H.; Latterini, L.; Rocha, S.; et al. Polymeric Engineering of Nanoparticles for Highly Efficient Multifunctional Drug Delivery Systems. Sci. Rep. 2019, 9, 2666. [Google Scholar] [CrossRef]
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R.; Zade, A.K.; Amani, A.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: A developing horizon. Nano Rev. Exp. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Zhao, L.; Shen, M.; Zhao, J.; Shi, X. A multifunctional polyethylenimine-based nanoplatform for targeted anticancer drug delivery to tumors in vivo. J. Mater. Chem. B 2017, 5, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Ihm, J.E.; Krier, I.; Lim, J.M.; Shim, S.; Han, D.K.; Hubbell, J.A. Improved biocompatibility of polyethylenimine (PEI) as a gene carrier by conjugating urocanic acid: In vitro and in vivo. Macromol. Res. 2015, 23, 387–395. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Meng, H.; Kabehie, S.; George, S.; Zink, J.I.; Nel, A.E. Polyethyleneimine Coating Enhances the Cellular Uptake of Mesoporous Silica Nanoparticles and Allows Safe Delivery of siRNA and DNA Constructs. ACS Nano 2009, 3, 3273–3286. [Google Scholar] [CrossRef]
- Hilgenbrink, A.R.; Low, P.S. Folate Receptor-Mediated Drug Targeting: From Therapeutics to Diagnostics. J. Pharm. Sci. 2005, 94, 2135–2146. [Google Scholar] [CrossRef]
- Jones, S.K.; Sarkar, A.; Feldmann, D.P.; Hoffmann, P.; Merkel, O. Revisiting the value of competition assays in folate receptor-mediated drug delivery. Biomaterials 2017, 138, 35–45. [Google Scholar] [CrossRef]
- Zwicke, G.L.; Mansoori, G.A.H.A.G.A.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3, 346. [Google Scholar] [CrossRef]
- Lao, J.; Madani, J.; Puértolas, T.; Álvarez, M.; Hernández, A.; Pazo-Cid, R.; Ángel, A.; Torres, A. A. Liposomal Doxorubicin in the Treatment of Breast Cancer Patients: A Review. J. Drug Deliv. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef] [Green Version]
- Trebunova, M.; Laputková, G.; Slabá, E.; Lacjakova, K.; Verebova, A. Effects of docetaxel, doxorubicin and cyclophosphamide on human breast cancer cell line MCF-7. Anticancer Res. 2012, 32, 2849–2854. [Google Scholar]
- Lawrie, T.A.; Rabbie, R.; Thoma, C.; Morrison, J. Pegylated liposomal doxorubicin for first-line treatment of epithelial ovarian cancer. Cochrane Database Syst. Rev. 2013, CD010482. [Google Scholar] [CrossRef]
- Nguyen, J.; Solimando, D.A.; Waddell, J.A. Carboplatin and Liposomal Doxorubicin for Ovarian Cancer. Hosp. Pharm. 2016, 51, 442–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisano, C.; Cecere, S.C.; Di Napoli, M.; Cavaliere, C.; Tambaro, R.; Facchini, G.; Scaffa, C.; Losito, S.; Pizzolorusso, A.; Pignata, S. Clinical Trials with Pegylated Liposomal Doxorubicin in the Treatment of Ovarian Cancer. J. Drug Deliv. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuokaya, W.; Kimura, T.; Miki, J.; Kimura, S.; Watanabe, H.; Bo, F.; Okada, D.; Aikawa, K.; Ochi, A.; Suzuki, K.; et al. Effectiveness of Intravesical Doxorubicin Immediately Following Resection of Primary Non–muscle-invasive Bladder Cancer: A Propensity Score-matched Analysis. Clin. Genitourin. Cancer 2020, 18, e55–e61. [Google Scholar] [CrossRef]
- Hong, Y.; Che, S.; Hui, B.; Yang, Y.; Wang, X.; Zhang, X.; Qiang, Y.; Ma, H. Lung cancer therapy using doxorubicin and curcumin combination: Targeted prodrug based, pH sensitive nanomedicine. Biomed. Pharmacother. 2019, 112, 108614. [Google Scholar] [CrossRef]
- Prados, J.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Caba, O.; Rama, A.R.; Delgado, A.V.; Arias, J. Enhanced antitumoral activity of doxorubicin against lung cancer cells using biodegradable poly(butylcyanoacrylate) nanoparticles. Drug Des. Dev. Ther. 2015, 9, 6433–6444. [Google Scholar] [CrossRef] [Green Version]
- Bey, E.A.; Bentle, M.S.; Reinicke, K.E.; Dong, Y.; Yang, C.-R.; Girard, L.; Minna, J.D.; Bornmann, W.G.; Gao, J.; Boothman, D.A. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc. Natl. Acad. Sci. USA 2007, 104, 11832–11837. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.I.; Choi, D.Y.; Chung, H.S.; Seo, H.G.; Woo, H.J.; Choi, B.T.; Choi, Y.H. beta-lapachone induces growth inhibition and apoptosis in bladder cancer cells by modulation of Bcl-2 family and activation of caspases. Exp. Oncol. 2006, 28, 30–35. [Google Scholar]
- Lin, M.T.; Chang, C.C.; Chen, S.-T.; Chang, H.-L.; Su, J.-L.; Chau, Y.-P.; Kuo, M.-L. Cyr61 expression confers resistance to apoptosis in breast cancer MCF-7 cells by a mechanism of NF-kappaB-dependent XIAP up-regulation. J. Biol. Chem. 2004, 279, 24015–24023. [Google Scholar] [CrossRef] [Green Version]
- Chau, Y.P.; Shiah, S.G.; Don, M.J.; Kuo, M.L. Involvement of hydrogen peroxide in topoisomerase inhibitor beta-lapachone-induced apoptosis and differentiation in human leukemia cells. Free Radic. Boil. Med. 1998, 24, 660–670. [Google Scholar] [CrossRef]
- Shiah, S.G.; Chuang, S.E.; Chau, Y.P.; Shen, S.C.; Kuo, M.L. Activation of c-Jun NH2-terminal kinase and subsequent CPP32/Yama during topoisomerase inhibitor beta-lapachone-induced apoptosis through an oxidation-dependent pathway. Cancer Res. 1999, 59, 391–398. [Google Scholar] [PubMed]
- Don, M.J.; Chang, Y.H.; Chen, K.K.; Ho, L.K.; Chau, Y.P. Induction of CDK inhibitors (p21(WAF1) and p27(Kip1)) and Bak in the beta-lapachone-induced apoptosis of human prostate cancer cells. Mol. Pharm. 2001, 59, 784–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.-J.; Lin, S.-Y.; Chau, Y.-P. Inhibition of poly(ADP-ribose) polymerase activation attenuates beta-lapachone-induced necrotic cell death in human osteosarcoma cells. Toxicol. Appl. Pharmacol. 2002, 182, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Liu, T.J.; Ho, L.K.; Don, M.J.; Chau, Y.P. beta-Lapachone induced cell death in human hepatoma (HepA2) cells. Histol. Histopathol. 1998, 13, 89–97. [Google Scholar] [PubMed]
- Li, L.S.; Bey, E.A.; Dong, Y.; Meng, J.; Patra, B.; Yan, J.; Xie, X.-J.; Brekken, R.A.; Barnett, C.C.; Bornmann, W.G.; et al. Modulating endogenous NQO1 levels identifies key regulatory mechanisms of action of β-lapachone for pancreatic cancer therapy. Clin. Cancer Res. 2011, 17, 275–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, G.W.; Ghaneh, P.; Costello, E.; Greenhalf, W.; Neoptolemos, J.P. New treatment options for advanced pancreatic cancer. Expert Rev. Gastroenterol. Hepatol. 2008, 2, 673–696. [Google Scholar] [CrossRef]
- Ough, M.; Lewis, A.; Bey, E.A.; Gao, J.; Ritchie, J.M.; Bornmann, W.; Boothman, D.A.; Oberley, L.W.; Cullen, J.J. Efficacy of beta-lapachone in pancreatic cancer treatment: Exploiting the novel, therapeutic target NQO1. Cancer Boil. Ther. 2005, 4, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altmana, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Silvers, M.A.; Deja, S.; Singh, N.; Egnatchik, R.A.; Sudderth, J.; Luo, X.; Beg, M.S.; Burgess, S.C.; DeBerardinis, R.J.; Boothman, D.A.; et al. The NQO1 bioactivatable drug, β-Lapachone, alters the redox state ofNQO1+ pancreatic cancer cells, causing perturbation in central carbon metabolism. J. Biol. Chem. 2017, 292, 18203–18216. [Google Scholar] [CrossRef] [Green Version]
- Giraudeau, P.; Baguet, E. Improvement of the inverse-gated-decoupling sequence for a faster quantitative analysis of various samples by 13C NMR spectroscopy. J. Magn. Reson. 2006, 180, 110–117. [Google Scholar] [CrossRef]
- Otte, D.A.L.; Borchmann, R.E.; Lin, C.; Weck, M.; Woerpel, K.A. 13C NMR Spectroscopy for the Quantitative Determination of Compound Ratios and Polymer End Groups. Org. Lett. 2014, 16, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, Z.; Song, X.; Cui, X.; Cao, P.; Liu, H.-J.; Cheng, F.; Chen, Y. Core-shell type multiarm star poly(ε-caprolactone) with high molecular weight hyperbranched polyethylenimine as core: Synthesis, characterization and encapsulation properties. Eur. Polym. J. 2008, 44, 1060–1070. [Google Scholar] [CrossRef]
- Li, J.; Xu, S.; Zheng, J.; Pan, Y.; Wang, J.; Zhang, L.; He, X.; Liu, D. Polypeptide-based star-block quadripolymers as unimolecular nanocarriers for the simultaneous encapsulation of hydrophobic and hydrophilic guests. Eur. Polym. J. 2012, 48, 1696–1708. [Google Scholar] [CrossRef]
- Liu, H.; Shen, Z.; Stiriba, S.-E.; Chen, Y.; Zhang, W.; Wei, L. Core–shell-type multiarm star polyethylenimine-block-poly(ε-caprolactone): Synthesis and guest encapsulation potential. J. Polym. Sci. A Polym. Chem. 2006, 44, 4165–4173. [Google Scholar] [CrossRef]
- Kasprzak, A.; Grudzinski, I.P.; Bamburowicz-Klimkowska, M.; Parzonko, A.; Gawlak, M.; Poplawska, M. New insight into the synthesis and biological activity of the polymeric materials consisting of folic acid and β-cyclodextrin. Macromol. Biosci. 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Bekers, O.; Beijnen, J.; Otagiri, M.; Bult, A.; Underberg, W. Inclusion complexation of doxorubicin and daunorubicin with cyclodextrins. J. Pharm. Biomed. Anal. 1990, 8, 671–674. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nasongkla, N.; Wiedmann, A.F.; Bruening, A.; Beman, M.; Ray, D.; Bornmann, W.G.; Boothman, D.A.; Gao, J. Enhancement of solubility and bioavailability of β-lapachone using cyclodextrin inclusion complexes. Pharm. Res. 2003, 20, 1626–1633. [Google Scholar]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug delivery: Is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjugate Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science. Principles and Applications; Academic Press: London, UK, 1988. [Google Scholar]
- Kratz, F.; Müller, I.A.; Ryppa, C.; Warnecke, A. Prodrug Strategies in Anticancer Chemotherapy. ChemMedChem 2008, 3, 20–53. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Richardson, S.C. Endocytosis and Intracellular Trafficking as Gateways for Nanomedicine Delivery: Opportunities and Challenges. Mol. Pharm. 2012, 9, 2380–2402. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Zhu, D.; Li, S.; Yu, X.; Zhao, Y.; Ouyang, X.; Xie, Z.; Li, L. Transporting carriers for intracellular targeting delivery via non-endocytic uptake pathways. Drug Deliv. 2017, 24, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Panchagnula, R. Review skeletal drug delivery systems. Int. J. Pharm. 2000, 206, 1–12. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Tannock, I.F.; Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar]
- Ahmed, S.A.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- Costa, P.C.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]









| Media | Higuchi | Korsmeyer–Peppas | ||
|---|---|---|---|---|
| kH [μg/h−0.5] | r2 | n | r2 | |
| PEI-βCD(DOX)-αCD(beta-LP)-FA | ||||
| pH 5.5 | 16.9 | 0.966 | 0.471 | 0.968 |
| pH 4.0 | 56.3 | 0.983 | 0.720 | 0.928 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, A.; Kasprzak, A.; Poplawska, M.; Ruzycka, M.; Grudzinski, I.P.; Nowicka, A.M. Controlled Drug Release and Cytotoxicity Studies of Beta-Lapachone and Doxorubicin Loaded into Cyclodextrins Attached to a Polyethyleneimine Matrix. Int. J. Mol. Sci. 2020, 21, 5832. https://doi.org/10.3390/ijms21165832
Kowalczyk A, Kasprzak A, Poplawska M, Ruzycka M, Grudzinski IP, Nowicka AM. Controlled Drug Release and Cytotoxicity Studies of Beta-Lapachone and Doxorubicin Loaded into Cyclodextrins Attached to a Polyethyleneimine Matrix. International Journal of Molecular Sciences. 2020; 21(16):5832. https://doi.org/10.3390/ijms21165832
Chicago/Turabian StyleKowalczyk, Agata, Artur Kasprzak, Magdalena Poplawska, Monika Ruzycka, Ireneusz P. Grudzinski, and Anna M. Nowicka. 2020. "Controlled Drug Release and Cytotoxicity Studies of Beta-Lapachone and Doxorubicin Loaded into Cyclodextrins Attached to a Polyethyleneimine Matrix" International Journal of Molecular Sciences 21, no. 16: 5832. https://doi.org/10.3390/ijms21165832
APA StyleKowalczyk, A., Kasprzak, A., Poplawska, M., Ruzycka, M., Grudzinski, I. P., & Nowicka, A. M. (2020). Controlled Drug Release and Cytotoxicity Studies of Beta-Lapachone and Doxorubicin Loaded into Cyclodextrins Attached to a Polyethyleneimine Matrix. International Journal of Molecular Sciences, 21(16), 5832. https://doi.org/10.3390/ijms21165832