Insights into the Role of Bioactive Food Ingredients and the Microbiome in Idiopathic Pulmonary Fibrosis
Abstract
:1. Introduction
2. Pathogenesis of IPF
Contribution of Aging Hallmarks to IPF
3. The Role of Nutritional Factors in IPF
3.1. Nutrition and Aging
3.2. Bioactive Food Ingredients Influencing IPF
3.2.1. Macronutrients
Lipids
Saturated Fatty Acids (SFA)
Polyunsaturated Fatty Acids (PUFA)
Carbohydrates
Amino Acids, Amino Acid Derivatives and Peptides
3.2.2. Micronutrients
Vitamins
- (1)
- Vitamin A
- (2)
- Vitamin B
- (3)
- Vitamin C
- (4)
- Vitamin D
- (5)
- Vitamin E
Minerals and Salt
- (1)
- Iron
- (2)
- Copper
- (3)
- Sodium Chloride
3.2.3. Phytochemicals
Quercetin
Curcumin
Resveratrol
Flaxseed Lignans and Schisandrin B
Epigallocatechin-3-Gallate
S-allyl-Compounds
| Phytochemical Compound | Dosage | Animal Models | Main Outcomes Related to Oxidative Stress, Inflammation, EMT and Fibrosis |
|---|---|---|---|
| Quercetin [111,141,142,143] | 10–100 mg/kg bw/day 800 mg/kg in diet | BLM and amiodarone-induced female and male mice and rats | ↓ MDA levels; ↑ Nrf2, CAT and SOD levels |
| ↓ TNFα, iNOS, IL-13/6, PDGF-β, levels; ↓ H&E staining; ↓ inflammatory cells; ↑ IFN-δ levels | |||
| ↓ COL1A2, TGF-β, fibronectin 1, pERK and MMP7 levels; ↓ hydroxyproline content; ↓ Masson’s trichrome staining | |||
| Curcumin [115,116,117,118,119,120,144] | 74–300mg/kg bw/day 1–5% w/w in diet | Irradiation, paraquat, BLM and amiodarone-induced female and male mice and rats | ↓ MPO activity; ↓ TBARS, GST and ROS levels; ↑ cathepsin K and L expression |
| ↓ NAG, AKP and ACE levels; ↓ c-Jun expression; ↓ TNF-α, superoxide anion and NO release; ↓ mononuclear and PMN cells | |||
| ↓ TGF-β1, α-SMA, hydroxyproline, type I collagen expression; ↓ Smad2-3 and ERK1/2 phosphorylation | |||
| Resveratrol [122,126,145] | 50 and 100 mg/kg bw/2 days 10 mg/kg bw/day 150 mg GSE/kg bw/day | BLM, silica, and particulate matter-induced male rats and mice | ↓ MDA levels; ↓ MPO activity; ↑ GSH levels |
| ↓ IL-6/1-β, TGF-β, TNF- α, NLRP3, ASC and caspase-1 levels; ↓ neutrophils; ↓ H&E staining | |||
| ↓ hydroxyproline and collagen content; ↓ Masson’s trichrome staining | |||
| Schisandrin B and flaxseed lignans [51,52,128,129,130,131,132] | 5–100 mg lignan/kg bw/day 2 mg flaxseed oil/kg bw/day 10–20% lignans, 10% flaxseed (w/w) and 15% flaxseed oil in diet | Irradiation and BLM-induced male and female mice and male rats | ↓ MDA, TBARS and Nox4 levels; ↓ nitrotyrosine staining; ↑ CAT and SOD activity |
| ↓ Alveolar PMN and macrophage influx, ↓ IL-1β/2/4/6/12/17, MIP-1α, VEGF, TNF-α and FGF levels | |||
| ↓ TGF-β, MMP7, β-catenin and hydroxyproline levels; ↓ Bax, p21, Smad2 phosphorylation | |||
| SAC and SAMC [136,138,139,140] | 25–200 mg/kg in bw/day 2 mL AGE/kg bw/2 days 0.15% in diet | TiO2, BLM and CCl4-induced male rats and male mice | ↓ Nox4 and LPO levels; ↑ HO-1, GSH and SOD activity; ↑ Nrf2 and thiol levels |
| ↓ TNF-α, IL-6 and iNOS levels; ↓ H&E staining; ↓ lymphocyte aggregation | |||
| ↓ TGF-β1, ↓ Smad3/P-Smad3, Smad2/P-Smad2 levels; ↓ MMP-9, TIMP-1, α-SMA, fibronectin, collagen 1A1 and collagen III expression; ↓ hydroxyproline content; ↓ Azan-Mallory staining | |||
| Astaxanthin [146,147,148] | 0.5, 1 and 2 mg/kg bw/day | BLM-induced rats | ↑ SOD and CAT activity |
| ↓ H&E staining | |||
| ↓ Hydroxyproline, collagen, vimentin and α-SMA levels; ↓ Masson’s trichrome staining; ↑ E-cadherin levels | |||
| Crocin [149,150] | 20 and 25 mg/kg bw/day | BLM-induced male rats | ↓ MDA and HO-1 levels; ↑ GSH and Nrf2 levels; ↑ GSH-px, SOD and CAT activity |
| ↓ NO, IL-10, TLR4 and TNF-α levels; ↓ H&E staining; ↓ total inflammatory cell, lymphocyte and neutrophil | |||
| ↓ Hydroxyproline content; ↓ Masson’s trichrome staining | |||
| Lycopene [151] | 5 mg/kg bw/day | BLM-induced male rats | ↓ MDA levels; ↑ SOD activity |
| ↓ H&E staining; NO and TNF-α levels | |||
| ↓ Masson’s trichrome staining | |||
| Zingerone [152] | 50 and 100 mg/kg bw/day | BLM-induced male rats | ↓ MDA levels; ↑ SOD and GSH-px activity |
| ↓ TNFα and IL-1β levels; ↓ H&E and iNOS staining | |||
| ↓ TGF-β1 expression; ↓ hydroxyproline content | |||
| Ellagic acid [153] | 15 mg/kg bw/day | BLM and cyclophosphamide-induced male rats | ↓ Lipid peroxidation; ↓ protein oxidation; ↓ NADH oxidize; ↓ MPO activity; ↑ CAT, SOD and GST activity |
| ↓ NO production | |||
| ↓ Hydroxyproline content | |||
| Proanthocyanidin [154] | 100 mg/kg bw/day | BLM-induced male rats | ↓ H&E and iNOS staining; ↓ immune system cells accumulation |
| ↓ Hydroxyproline content |
4. The Role of Human Microbiota in IPF—An Emerging Therapeutic Strategy
4.1. Bacterial Burden, Diversity and Microbial Composition in the Lung in IPF
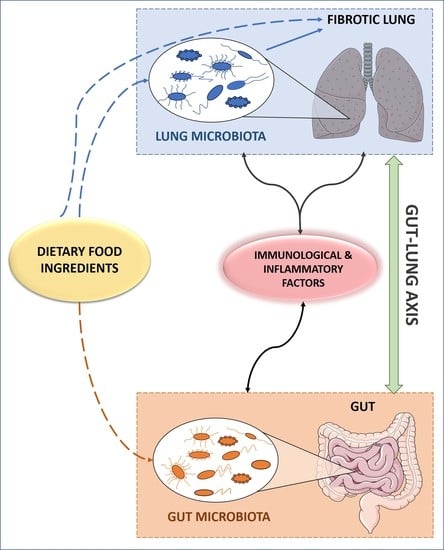
4.2. The Gut-Lung Axis: Interplay of the Gut and Lung Microbiota in IPF
Connecting Lung and Gut Microbiota with The Immune System and Inflammation: An Inter-organ Cross Talk
5. Bioactive Food Ingredients, Microbiota and IPF
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Sauleda, J.; Núñez, B.; Sala, E.; Soriano, J.B. Idiopathic Pulmonary Fibrosis: Epidemiology, Natural History, Phenotypes. Med. Sci. (Basel) 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Calyeca, J.; Rojas, M.; Mora, A.L. Mitochondria dysfunction and metabolic reprogramming as drivers of idiopathic pulmonary fibrosis. Redox Biol. 2020, 33, 101509. [Google Scholar] [CrossRef] [PubMed]
- Cárdenes, N.; Álvarez, D.; Sellarés, J.; Peng, Y.; Corey, C.; Wecht, S.; Nouraie, S.M.; Shanker, S.; Sembrat, J.; Bueno, M.; et al. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res. Ther. 2018, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Planas-Cerezales, L.; Arias-Salgado, E.G.; Buendia-Roldán, I.; Montes-Worboys, A.; López, C.E.; Vicens-Zygmunt, V.; Hernaiz, P.L.; Sanuy, R.L.; Leiro-Fernandez, V.; Vilarnau, E.B.; et al. Predictive factors and prognostic effect of telomere shortening in pulmonary fibrosis. Respirology 2019, 24, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Wolters, P.J.; Collard, H.R.; Jones, K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 2014, 9, 157–179. [Google Scholar] [CrossRef] [Green Version]
- Sunaga, H.; Matsui, H.; Ueno, M.; Maeno, T.; Iso, T.; Syamsunarno, M.R.; Anjo, S.; Matsuzaka, T.; Shimano, H.; Yokoyama, T.; et al. Deranged fatty acid composition causes pulmonary fibrosis in Elovl6-deficient mice. Nat. Commun. 2013, 4, 2563. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.J.; Moon, J.S.; Lee, C.M.; Choi, A.M.; Stout-Delgado, H.W. Glucose Transporter 1-Dependent Glycolysis Is Increased during Aging-Related Lung Fibrosis, and Phloretin Inhibits Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2017, 56, 521–531. [Google Scholar] [CrossRef]
- Zhao, H.; Dennery, P.A.; Yao, H. Metabolic reprogramming in the pathogenesis of chronic lung diseases, including BPD, COPD, and pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L544–L554. [Google Scholar] [CrossRef]
- O’Dwyer, D.N.; Ashley, S.L.; Gurczynski, S.J.; Xia, M.; Wilke, C.; Falkowski, N.R.; Norman, K.C.; Arnold, K.B.; Huffnagle, G.B.; Salisbury, M.L.; et al. Lung Microbiota Contribute to Pulmonary Inflammation and Disease Progression in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Molyneaux, P.L.; Cox, M.J.; Willis-Owen, S.A.; Mallia, P.; Russell, K.E.; Russell, A.M.; Murphy, E.; Johnston, S.L.; Schwartz, D.A.; Wells, A.U.; et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2014, 190, 906–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molyneaux, P.L.; Willis-Owen, S.A.G.; Cox, M.J.; James, P.; Cowman, S.; Loebinger, M.; Blanchard, A.; Edwards, L.M.; Stock, C.; Daccord, C.; et al. Host-Microbial Interactions in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 195, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Struman, I.; Chavez, V.; Henket, M.; Herzog, M.; Scoubeau, K.; Hardat, N.; Bondue, B.; Corhay, J.L.; Moermans, C.; et al. Altered epigenetic features in circulating nucleosomes in idiopathic pulmonary fibrosis. Clin. Epigenetics 2017, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Jones, M.G.; Davies, D.E.; Wang, Y. Epithelial-mesenchymal transition contributes to pulmonary fibrosis via aberrant epithelial/fibroblastic cross-talk. J. Lung Health Dis. 2019, 3, 31–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drakopanagiotakis, F.; Wujak, L.; Wygrecka, M.; Markart, P. Biomarkers in idiopathic pulmonary fibrosis. Matrix Biol. 2018, 68–69, 404–421. [Google Scholar] [CrossRef]
- Cameli, P.; Carleo, A.; Bergantini, L.; Landi, C.; Prasse, A.; Bargagli, E. Oxidant/Antioxidant Disequilibrium in Idiopathic Pulmonary Fibrosis Pathogenesis. Inflammation 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Otoupalova, E.; Smith, S.; Cheng, G.; Thannickal, V.J. Oxidative Stress in Pulmonary Fibrosis. Compr. Physiol. 2020, 10, 509–547. [Google Scholar] [CrossRef] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Schuliga, M.; Pechkovsky, D.V.; Read, J.; Waters, D.W.; Blokland, K.E.C.; Reid, A.T.; Hogaboam, C.M.; Khalil, N.; Burgess, J.K.; Prêle, C.M.; et al. Mitochondrial dysfunction contributes to the senescent phenotype of IPF lung fibroblasts. J. Cell Mol. Med. 2018, 22, 5847–5861. [Google Scholar] [CrossRef]
- Jayedi, A.; Soltani, S.; Abdolshahi, A.; Shab-Bidar, S. Healthy and Unhealthy Dietary Patterns and the Risk of Chronic Disease: An Umbrella Review of Meta-Analyses of Prospective Cohort Studies. Br. J. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional Considerations for Healthy Aging and Reduction in Age-Related Chronic Disease. Adv. Nutr. 2017, 8, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, I.R.; Ames, R.W.; Evans, M.C.; Gamble, G.D.; Sharpe, S.J. Effect of calcium supplementation on bone loss in postmenopausal women. N. Engl. J. Med. 1993, 328, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Gielen, E.; Beckwée, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I.; Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG). Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutr. Rev. 2020. [Google Scholar] [CrossRef]
- Bamia, C.; Trichopoulos, D.; Ferrari, P.; Overvad, K.; Bjerregaard, L.; Tjønneland, A.; Halkjaer, J.; Clavel-Chapelon, F.; Kesse, E.; Boutron-Ruault, M.C.; et al. Dietary patterns and survival of older Europeans: The EPIC-Elderly Study (European Prospective Investigation into Cancer and Nutrition). Public Health Nutr. 2007, 10, 590–598. [Google Scholar] [CrossRef] [Green Version]
- Cava, E.; Fontana, L. Will calorie restriction work in humans? Aging (Albany NY) 2013, 5, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Vaiserman, A.; Lushchak, O. Implementation of longevity-promoting supplements and medications in public health practice: Achievements, challenges and future perspectives. J. Transl. Med. 2017, 15, 160. [Google Scholar] [CrossRef]
- Hegab, A.E.; Ozaki, M.; Meligy, F.Y.; Nishino, M.; Kagawa, S.; Ishii, M.; Betsuyaku, T. Calorie restriction enhances adult mouse lung stem cells function and reverses several ageing-induced changes. J. Tissue Eng. Regen. Med. 2019, 13, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Hegab, A.E.; Ozaki, M.; Meligy, F.Y.; Kagawa, S.; Ishii, M.; Betsuyaku, T. High fat diet activates adult mouse lung stem cells and accelerates several aging-induced effects. Stem Cell Res. 2018, 33, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Heyob, K.M.; Mieth, S.; Sugar, S.S.; Graf, A.E.; Lallier, S.W.; Britt, R.D.; Rogers, L.K. Maternal high-fat diet alters lung development and function in the offspring. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L167–L174. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Yokoyama, T.; Chida, K.; Azuma, A.; Suda, T.; Kudoh, S.; Sakamoto, N.; Okamoto, K.; Kobashi, G.; et al. Vegetable, fruit, and cereal intake and risk of idiopathic pulmonary fibrosis in Japan. Ann. Nutr. Metab 2004, 48, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Yokoyama, T.; Chida, K.; Azuma, A.; Suda, T.; Kudoh, S.; Sakamoto, N.; Okamoto, K.; Kobashi, G.; et al. Dietary fat and meat intake and idiopathic pulmonary fibrosis: A case-control study in Japan. Int. J. Tuberc. Lung Dis. 2006, 10, 333–339. [Google Scholar] [PubMed]
- Schmidt, R.; Meier, U.; Markart, P.; Grimminger, F.; Velcovsky, H.G.; Morr, H.; Seeger, W.; Günther, A. Altered fatty acid composition of lung surfactant phospholipids in interstitial lung disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L1079–L1085. [Google Scholar] [CrossRef] [Green Version]
- Jouneau, S.; Lederlin, M.; Vernhet, L.; Thibault, R. Malnutrition in idiopathic pulmonary fibrosis: The great forgotten comorbidity! Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef]
- Faverio, P.; Bocchino, M.; Caminati, A.; Fumagalli, A.; Gasbarra, M.; Iovino, P.; Petruzzi, A.; Scalfi, L.; Sebastiani, A.; Stanziola, A.A.; et al. Nutrition in Patients with Idiopathic Pulmonary Fibrosis: Critical Issues Analysis and Future Research Directions. Nutrients 2020, 12, 1131. [Google Scholar] [CrossRef] [Green Version]
- Iannello, S.; Cavaleri, A.; Camuto, M.; Pisano, M.G.; Milazzo, P.; Belfiore, F. Low fasting serum triglyceride and high free fatty acid levels in pulmonary fibrosis: A previously unreported finding. MedGenMed 2002, 4, 5. [Google Scholar]
- Ye, Z.; Huang, Y.; Liu, D.; Chen, X.; Wang, D.; Huang, D.; Zhao, L.; Xiao, X. Obesity induced by neonatal overfeeding worsens airway hyperresponsiveness and inflammation. PLoS ONE 2012, 7, e47013. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.H.; Oh, E.Y.; Han, H.; Yang, M.; Park, H.J.; Park, K.H.; Lee, J.H.; Park, J.W. Insulin resistance mediates high-fat diet-induced pulmonary fibrosis and airway hyperresponsiveness through the TGF-β1 pathway. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.N.; Greenberg, Y.; Hosseinkhani, M.R.; Long, E.K.; Bahaie, N.S.; Rao, A.; Ha, S.G.; Rao, S.P.; Bernlohr, D.A.; Sriramarao, P. High-fat diet promotes lung fibrosis and attenuates airway eosinophilia after exposure to cockroach allergen in mice. Exp. Lung Res. 2013, 39, 365–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vedova, M.C.D.; Soler Garcia, F.M.; Muñoz, M.D.; Fornes, M.W.; Gomez Mejiba, S.E.; Gómez, N.N.; Ramirez, D.C. Diet-Induced Pulmonary Inflammation and Incipient Fibrosis in Mice: A Possible Role of Neutrophilic Inflammation. Inflammation 2019, 42, 1886–1900. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.D.; Yin, L.; Archer, S.; Lu, C.; Zhao, G.; Yao, Y.; Wu, L.; Hsin, M.; Waddell, T.K.; Keshavjee, S.; et al. Metabolic heterogeneity of idiopathic pulmonary fibrosis: A metabolomic study. BMJ Open Respir. Res. 2017, 4, e000183. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Wen, Z.; Wang, R.; Luo, W.; Du, Y.; Wang, W.; Chen, X. Identification of the lipid biomarkers from plasma in idiopathic pulmonary fibrosis by Lipidomics. BMC Pulm. Med. 2017, 17, 174. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.I.; Chandler, D.B.; Fulmer, J.D.; Wert, M.B.; Grizzle, W.E. Effects of dietary fats on bleomycin-induced pulmonary fibrosis. Exp. Lung Res. 1987, 12, 149–161. [Google Scholar] [CrossRef]
- Chu, S.G.; Villalba, J.A.; Liang, X.; Xiong, K.; Tsoyi, K.; Ith, B.; Ayaub, E.A.; Tatituri, R.V.; Byers, D.E.; Hsu, F.F.; et al. Palmitic Acid-Rich High-Fat Diet Exacerbates Experimental Pulmonary Fibrosis by Modulating Endoplasmic Reticulum Stress. Am. J. Respir. Cell Mol. Biol. 2019, 61, 737–746. [Google Scholar] [CrossRef]
- Kennedy, J.I.; Chandler, D.B.; Fulmer, J.D.; Wert, M.B.; Grizzle, W.E. Dietary fish oil inhibits bleomycin-induced pulmonary fibrosis in the rat. Exp. Lung Res. 1989, 15, 315–329. [Google Scholar] [CrossRef]
- Velten, M.; Britt, R.D.; Heyob, K.M.; Tipple, T.E.; Rogers, L.K. Maternal dietary docosahexaenoic acid supplementation attenuates fetal growth restriction and enhances pulmonary function in a newborn mouse model of perinatal inflammation. J. Nutr. 2014, 144, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zeng, T.; Zhao, X.; Xiea, K.; Bi, Y.; Zhong, Z. Docosahexaenoic acid (DHA) ameliorates paraquat-induced pulmonary fibrosis in rats possibly through up-regulation of Smad 7 and SnoN. Food Chem. Toxicol. 2013, 57, 330–337. [Google Scholar] [CrossRef]
- Lawrenz, J.; Herndon, B.; Kamal, A.; Mehrer, A.; Dim, D.C.; Baidoo, C.; Gasper, D.; Nitz, J.; Molteni, A.; Baybutt, R.C. Dietary Flaxseed Oil Protects against Bleomycin-Induced Pulmonary Fibrosis in Rats. Pulm. Med. 2012, 2012, 457031. [Google Scholar] [CrossRef] [PubMed]
- Abidi, A.; Kourda, N.; Feki, M.; Ben Khamsa, S. Protective Effect of Tunisian Flaxseed Oil against Bleomycin-Induced Pulmonary Fibrosis in Rats. Nutr. Cancer 2020, 72, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Ziboh, V.A.; Yun, M.; Hyde, D.M.; Giri, S.N. gamma-Linolenic acid-containing diet attenuates bleomycin-induced lung fibrosis in hamsters. Lipids 1997, 32, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, Q.; D’Souza, V.; Bartis, D.; Dancer, R.; Parekh, D.; Gao, F.; Lian, Q.; Jin, S.; Thickett, D.R. ResolvinD. Lab. Invest. 2018, 98, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.T.; Lakshmi, S.P.; Zhang, Y.; Reddy, R.C. Nitrated fatty acids reverse pulmonary fibrosis by dedifferentiating myofibroblasts and promoting collagen uptake by alveolar macrophages. FASEB J. 2014, 28, 5299–5310. [Google Scholar] [CrossRef] [Green Version]
- Iraz, M.; Bilgic, S.; Samdanci, E.; Ozerol, E.; Tanbek, K. Preventive and early therapeutic effects of β-glucan on the bleomycin-induced lung fibrosis in rats. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1505–1516. [Google Scholar]
- Zhou, S.; Zhou, Y.; Yu, J.; Du, Y.; Tan, Y.; Ke, Y.; Wang, J.; Han, B.; Ge, F. Ophiocordyceps lanpingensis polysaccharides attenuate pulmonary fibrosis in mice. Biomed. Pharmacother. 2020, 126, 110058. [Google Scholar] [CrossRef]
- Chen, J.; Shi, Y.; He, L.; Hao, H.; Wang, B.; Zheng, Y.; Hu, C. Protective roles of polysaccharides from Ganoderma lucidum on bleomycin-induced pulmonary fibrosis in rats. Int. J. Biol. Macromol. 2016, 92, 278–281. [Google Scholar] [CrossRef]
- Yu, H.H.; Chengchuan Ko, E.; Chang, C.L.; Yuan, K.S.; Wu, A.T.H.; Shan, Y.S.; Wu, S.Y. Fucoidan Inhibits Radiation-Induced Pneumonitis and Lung Fibrosis by Reducing Inflammatory Cytokine Expression in Lung Tissues. Mar. Drugs 2018, 16, 392. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Li, Q.; Youn, H.Y.; Kim, D.Y. Oral Administration of Chitosan Attenuates Bleomycin-induced Pulmonary Fibrosis in Rats. In Vivo 2019, 33, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.L.; He, X.Y.; Xu, F.Y.; Du, B.X.; Zou, Z.; Shi, X.Y. Chitosan aerosol inhalation alleviates lipopolysaccharide- induced pulmonary fibrosis in rats. Exp. Lung Res. 2014, 40, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, J.; Wang, B.; Zhang, X.; Huang, Q.; Yuan, J.; Hao, H.; Chen, X.; Zhi, J.; Zhao, L.; et al. Polysaccharides from Dendrobium officinale inhibit bleomycin-induced pulmonary fibrosis via the TGFβ1-Smad2/3 axis. Int. J. Biol. Macromol. 2018, 118, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, L.C.; Zhao, P.; Qi, L.W.; Li, P.; Gao, J.; Fei, G.H. Total polysaccharide of Yupingfeng protects against bleomycin-induced pulmonary fibrosis via inhibiting transforming growth factor-β1-mediated type I collagen abnormal deposition in rats. J. Pharm. Pharmacol. 2014, 66, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Gaugg, M.T.; Engler, A.; Bregy, L.; Nussbaumer-Ochsner, Y.; Eiffert, L.; Bruderer, T.; Zenobi, R.; Sinues, P.; Kohler, M. Molecular breath analysis supports altered amino acid metabolism in idiopathic pulmonary fibrosis. Respirology 2019, 24, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Zhang, J.H.; Chen, X.X.; Ren, H.L.; Feng, X.L.; Wang, J.L.; Xiao, J.H. Combination of L-Arginine and L-Norvaline protects against pulmonary fibrosis progression induced by bleomycin in mice. Biomed. Pharmacother 2019, 113, 108768. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, D.; Cui, X.; Hu, W. The protective action of taurine and L-arginine in radiation pulmonary fibrosis. J. Environ. Pathol. Toxicol. Oncol. 1998, 17, 151–157. [Google Scholar]
- Ma, X.; Zhang, Y.; Jiang, D.; Yang, Y.; Wu, G.; Wu, Z. Protective Effects of Functional Amino Acids on Apoptosis, Inflammatory Response, and Pulmonary Fibrosis in Lipopolysaccharide-Challenged Mice. J. Agric. Food Chem. 2019, 67, 4915–4922. [Google Scholar] [CrossRef]
- Giri, S.N.; Wang, Q. Taurine and niacin offer a novel therapeutic modality in prevention of chemically-induced pulmonary fibrosis in hamsters. Adv. Exp. Med. Biol. 1992, 315, 329–340. [Google Scholar] [CrossRef]
- Wang, Q.J.; Giri, S.N.; Hyde, D.M.; Nakashima, J.M. Effects of taurine on bleomycin-induced lung fibrosis in hamsters. Proc. Soc. Exp. Biol. Med. 1989, 190, 330–338. [Google Scholar] [CrossRef]
- Wang, Q.J.; Giri, S.N.; Hyde, D.M.; Nakashima, J.M.; Javadi, I. Niacin attenuates bleomycin-induced lung fibrosis in the hamster. J. Biochem. Toxicol. 1990, 5, 13–22. [Google Scholar] [CrossRef]
- Gurujeyalakshmi, G.; Hollinger, M.A.; Giri, S.N. Regulation of transforming growth factor-beta1 mRNA expression by taurine and niacin in the bleomycin hamster model of lung fibrosis. Am. J. Respir. Cell Mol. Biol. 1998, 18, 334–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaisdell, R.J.; Giri, S.N. Mechanism of antifibrotic effect of taurine and niacin in the multidose bleomycin-hamster model of lung fibrosis: Inhibition of lysyl oxidase and collagenase. J. Biochem. Toxicol. 1995, 10, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Z.; Huang, H.; Wang, Y.; Lin, B.; Wu, S.; Ma, J.; Chen, B.; He, Z.; Wu, J.; et al. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the novel peptide EZY-1 purified from Eucheuma. Food Funct. 2019, 10, 3198–3208. [Google Scholar] [CrossRef] [PubMed]
- Gruchlik, A.; Chodurek, E.; Dzierzewicz, Z. Effect of GLY-HIS-LYS and its copper complex on TGF-β secretion in normal human dermal fibroblasts. Acta Pol. Pharm. 2014, 71, 954–958. [Google Scholar]
- Zhou, X.M.; Wang, G.L.; Wang, X.B.; Liu, L.; Zhang, Q.; Yin, Y.; Wang, Q.Y.; Kang, J.; Hou, G. GHK Peptide Inhibits Bleomycin-Induced Pulmonary Fibrosis in Mice by Suppressing TGFβ1/Smad-Mediated Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2017, 8, 904. [Google Scholar] [CrossRef] [Green Version]
- Markart, P.; Luboeinski, T.; Korfei, M.; Schmidt, R.; Wygrecka, M.; Mahavadi, P.; Mayer, K.; Wilhelm, J.; Seeger, W.; Guenther, A.; et al. Alveolar oxidative stress is associated with elevated levels of nonenzymatic low-molecular-weight antioxidants in patients with different forms of chronic fibrosing interstitial lung diseases. Antioxid. Redox Signal. 2009, 11, 227–240. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Enhancement of antioxidant defense system by epigallocatechin-3-gallate during bleomycin induced experimental pulmonary fibrosis. Biol. Pharm. Bull. 2008, 31, 1306–1311. [Google Scholar] [CrossRef] [Green Version]
- Tabata, C.; Kadokawa, Y.; Tabata, R.; Takahashi, M.; Okoshi, K.; Sakai, Y.; Mishima, M.; Kubo, H. All-trans-retinoic acid prevents radiation- or bleomycin-induced pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 1352–1360. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Tai, W.; Yang, Y.; Zhang, T.; Li, Y.; Chai, Y.; Zhong, H.; Zou, H.; Wang, D. The role of all-trans retinoic acid in bleomycin-induced pulmonary fibrosis in mice. Exp. Lung Res. 2012, 38, 82–89. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Nazari, Z.; Ranjbari, N.; Torfi, A. Comparison of the preventive effect of vitamin C and E on hexavalent chromium induced pulmonary fibrosis in rat. Inflammopharmacology 2008, 16, 195–197. [Google Scholar] [CrossRef]
- Rodrigues da Silva, M.; Schapochnik, A.; Peres Leal, M.; Esteves, J.; Bichels Hebeda, C.; Sandri, S.; Pavani, C.; Ratto Tempestini Horliana, A.C.; Farsky, S.H.P.; Lino-Dos-Santos-Franco, A. Beneficial effects of ascorbic acid to treat lung fibrosis induced by paraquat. PLoS ONE 2018, 13, e0205535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, H.A.; Elbastawisy, Y.M.; Elsaed, W.M. Attenuation of lipopolysaccharide-induced lung inflammation by ascorbic acid in rats: Histopathological and ultrastructural study. SAGE Open Med. 2019, 7, 2050312119828260. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Kennedy, T.P.; Crissman, K.M.; Richards, J.H.; Hatch, G.E. Depletion of iron and ascorbate in rodents diminishes lung injury after silica. Exp. Lung Res. 1998, 24, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Tzilas, V.; Bouros, E.; Barbayianni, I.; Karampitsakos, T.; Kourtidou, S.; Ntassiou, M.; Ninou, I.; Aidinis, V.; Bouros, D.; Tzouvelekis, A. Vitamin D prevents experimental lung fibrosis and predicts survival in patients with idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 2019, 55, 17–24. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Q.; Qiu, X.; Zhuang, Y.; Yu, M.; Dai, J.; Cai, H.; Yan, X. Vitamin D levels are prognostic factors for connective tissue disease associated interstitial lung disease (CTD-ILD). Aging (Albany NY) 2020, 12, 4371–4378. [Google Scholar] [CrossRef]
- Ma, D.; Peng, L. Vitamin D and pulmonary fibrosis: A review of molecular mechanisms. Int. J. Clin. Exp. Pathol. 2019, 12, 3171–3178. [Google Scholar]
- Zhang, Z.; Yu, X.; Fang, X.; Liang, A.; Yu, Z.; Gu, P.; Zeng, Y.; He, J.; Zhu, H.; Li, S.; et al. Preventive effects of vitamin D treatment on bleomycin-induced pulmonary fibrosis. Sci. Rep. 2015, 5, 17638. [Google Scholar] [CrossRef] [Green Version]
- Schapochnik, A.; da Silva, M.R.; Leal, M.P.; Esteves, J.; Hebeda, C.B.; Sandri, S.; de Fátima Teixeira da Silva, D.; Farsky, S.H.P.; Marcos, R.L.; Lino-Dos-Santos-Franco, A. Vitamin D treatment abrogates the inflammatory response in paraquat-induced lung fibrosis. Toxicol. Appl. Pharmacol. 2018, 355, 60–67. [Google Scholar] [CrossRef]
- Nakamura, H.; Sato, S.; Takahashi, K. Effects of vitamin E deficiency on bleomycin-induced pulmonary fibrosis in the hamster. Lung 1988, 166, 161–176. [Google Scholar] [CrossRef]
- Kilinç, C.; Ozcan, O.; Karaöz, E.; Sunguroğlu, K.; Kutluay, T.; Karaca, L. Vitamin E reduces bleomycin-induced lung fibrosis in mice: Biochemical and morphological studies. J. Basic Clin. Physiol. Pharmacol. 1993, 4, 249–269. [Google Scholar] [CrossRef]
- Denis, M. Antioxidant therapy partially blocks immune-induced lung fibrosis. Inflammation 1995, 19, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Card, J.W.; Leeder, R.G.; Racz, W.J.; Brien, J.F.; Bray, T.M.; Massey, T.E. Effects of dietary vitamin E supplementation on pulmonary morphology and collagen deposition in amiodarone- and vehicle-treated hamsters. Toxicology 1999, 133, 75–84. [Google Scholar] [CrossRef]
- Bese, N.S.; Munzuroglu, F.; Uslu, B.; Arbak, S.; Yesiladali, G.; Sut, N.; Altug, T.; Ober, A. Vitamin E protects against the development of radiation-induced pulmonary fibrosis in rats. Clin. Oncol. (R Coll Radiol) 2007, 19, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Wigenstam, E.; Rocksén, D.; Ekstrand-Hammarström, B.; Bucht, A. Treatment with dexamethasone or liposome-encapsuled vitamin E provides beneficial effects after chemical-induced lung injury. Inhal. Toxicol. 2009, 21, 958–964. [Google Scholar] [CrossRef]
- Delanian, S.; Balla-Mekias, S.; Lefaix, J.L. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J. Clin. Oncol. 1999, 17, 3283–3290. [Google Scholar] [CrossRef]
- Ogger, P.P.; Byrne, A.J. Lung fibrosis enters the iron age. J. Pathol. 2020. [Google Scholar] [CrossRef]
- Kim, K.H.; Maldonado, F.; Ryu, J.H.; Eiken, P.W.; Hartman, T.E.; Bartholmai, B.J.; Decker, P.A.; Yi, E.S. Iron deposition and increased alveolar septal capillary density in nonfibrotic lung tissue are associated with pulmonary hypertension in idiopathic pulmonary fibrosis. Respir. Res. 2010, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Puxeddu, E.; Comandini, A.; Cavalli, F.; Pezzuto, G.; D’Ambrosio, C.; Senis, L.; Paci, M.; Curradi, G.; Sergiacomi, G.L.; Saltini, C. Iron laden macrophages in idiopathic pulmonary fibrosis: The telltale of occult alveolar hemorrhage? Pulm. Pharmacol. Ther. 2014, 28, 35–40. [Google Scholar] [CrossRef]
- Lee, J.; Arisi, I.; Puxeddu, E.; Mramba, L.K.; Amicosante, M.; Swaisgood, C.M.; Pallante, M.; Brantly, M.L.; Sköld, C.M.; Saltini, C. Bronchoalveolar lavage (BAL) cells in idiopathic pulmonary fibrosis express a complex pro-inflammatory, pro-repair, angiogenic activation pattern, likely associated with macrophage iron accumulation. PLoS ONE 2018, 13, e0194803. [Google Scholar] [CrossRef]
- Ali, M.K.; Kim, R.Y.; Brown, A.C.; Donovan, C.; Vanka, K.S.; Mayall, J.R.; Liu, G.; Pillar, A.L.; Jones-Freeman, B.; Xenaki, D.; et al. Critical role for iron accumulation in the pathogenesis of fibrotic lung disease. J. Pathol. 2020, 251, 49–62. [Google Scholar] [CrossRef]
- Chandler, D.B.; Barton, J.C.; Briggs, D.D.; Butler, T.W.; Kennedy, J.I.; Grizzle, W.E.; Fulmer, J.D. Effect of iron deficiency on bleomycin-induced lung fibrosis in the hamster. Am. Rev. Respir. Dis. 1988, 137, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef] [Green Version]
- Janssen, R.; de Brouwer, B.; von der Thüsen, J.H.; Wouters, E.F.M. Copper as the most likely pathogenic divergence factor between lung fibrosis and emphysema. Med. Hypotheses 2018, 120, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Boulet, N.; Chaplin, A.; Mercader, J. Past, Present and Future Anti-Obesity Effects of Flavin-Containing and/or Copper-Containing Amine Oxidase Inhibitors. Medicines (Basel) 2019, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Marttila-Ichihara, F.; Elima, K.; Auvinen, K.; Veres, T.Z.; Rantakari, P.; Weston, C.; Miyasaka, M.; Adams, D.; Jalkanen, S.; Salmi, M. Amine oxidase activity regulates the development of pulmonary fibrosis. FASEB J. 2017, 31, 2477–2491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovet, H.; Oztay, F. The copper chelator tetrathiomolybdate regressed bleomycin-induced pulmonary fibrosis in mice, by reducing lysyl oxidase expressions. Biol. Trace. Elem. Res. 2014, 162, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Gomer, R.H. New approaches to modulating idiopathic pulmonary fibrosis. Curr. Allergy Asthma Rep. 2013, 13, 607–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, N.; Pilling, D.; Gomer, R.H. NaCl potentiates human fibrocyte differentiation. PLoS ONE 2012, 7, e45674. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Pilling, D.; Gomer, R.H. Dietary NaCl affects bleomycin-induced lung fibrosis in mice. Exp. Lung Res. 2017, 43, 395–406. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Wu, C.Y.; Peng, H.H.; Voisin, L.; Perfettini, J.L.; Ko, Y.F.; Young, J.D. Emerging use of senolytics and senomorphics against aging and chronic diseases. Med. Res. Rev. 2020. [Google Scholar] [CrossRef]
- Boots, A.W.; Veith, C.; Albrecht, C.; Bartholome, R.; Drittij, M.J.; Claessen, S.M.H.; Bast, A.; Rosenbruch, M.; Jonkers, L.; van Schooten, F.J.; et al. The dietary antioxidant quercetin reduces hallmarks of bleomycin-induced lung fibrogenesis in mice. BMC Pulm. Med. 2020, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, M.S.; Habiel, D.M.; Coelho, A.L.; Verri, W.A.; Hogaboam, C.M. Quercetin Enhances Ligand-induced Apoptosis in Senescent Idiopathic Pulmonary Fibrosis Fibroblasts and Reduces Lung Fibrosis In Vivo. Am. J. Respir. Cell Mol. Biol. 2019, 60, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelli, D.; Sahebkar, A.; Johnston, T.P.; Pedone, C. Curcumin use in pulmonary diseases: State of the art and future perspectives. Pharmacol. Res. 2017, 115, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Thresiamma, K.C.; George, J.; Kuttan, R. Protective effect of curcumin, ellagic acid and bixin on radiation induced toxicity. Indian J. Exp. Biol. 1996, 34, 845–847. [Google Scholar]
- Venkatesan, N. Pulmonary protective effects of curcumin against paraquat toxicity. Life Sci. 2000, 66, PL21–PL28. [Google Scholar] [CrossRef]
- Punithavathi, D.; Venkatesan, N.; Babu, M. Protective effects of curcumin against amiodarone-induced pulmonary fibrosis in rats. Br. J. Pharmacol. 2003, 139, 1342–1350. [Google Scholar] [CrossRef]
- Lee, J.C.; Kinniry, P.A.; Arguiri, E.; Serota, M.; Kanterakis, S.; Chatterjee, S.; Solomides, C.C.; Javvadi, P.; Koumenis, C.; Cengel, K.A.; et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat. Res. 2010, 173, 590–601. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Huang, C.; Yang, C.; Liu, R.J.; Wang, J.; Niu, J.; Brömme, D. Antifibrotic effects of curcumin are associated with overexpression of cathepsins K and L in bleomycin treated mice and human fibroblasts. Respir. Res. 2011, 12, 154. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.R.; Gangireddy, S.R.; Narala, V.R.; Hogaboam, C.M.; Standiford, T.J.; Christensen, P.J.; Kondapi, A.K.; Reddy, R.C. Curcumin inhibits fibrosis-related effects in IPF fibroblasts and in mice following bleomycin-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, L616–L625. [Google Scholar] [CrossRef]
- Hu, Y.; Li, M.; Zhang, M.; Jin, Y. Inhalation treatment of idiopathic pulmonary fibrosis with curcumin large porous microparticles. Int. J. Pharm. 2018, 551, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Topaloğlu, N.; Sehirli, A.O.; Ercan, F.; Gedik, N. Resveratrol alleviates bleomycin-induced lung injury in rats. Pulm. Pharmacol. Ther. 2007, 20, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Liu, Y.J.; Mao, Y.F.; Dong, W.W.; Zhu, X.Y.; Jiang, L. Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-β1 signaling. Clin. Nutr. 2015, 34, 752–760. [Google Scholar] [CrossRef]
- Fagone, E.; Conte, E.; Gili, E.; Fruciano, M.; Pistorio, M.P.; Lo Furno, D.; Giuffrida, R.; Crimi, N.; Vancheri, C. Resveratrol inhibits transforming growth factor-β-induced proliferation and differentiation of ex vivo human lung fibroblasts into myofibroblasts through ERK/Akt inhibition and PTEN restoration. Exp. Lung Res. 2011, 37, 162–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, L.; Wu, J.; Wang, W.; Zhao, R.P.; Xu, X.W.; Hu, D. Sirt 1 activator attenuates the bleomycin-induced lung fibrosis in mice via inhibiting epithelial-to-mesenchymal transition (EMT). Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2144–2150. [Google Scholar]
- Ding, S.; Wang, H.; Wang, M.; Bai, L.; Yu, P.; Wu, W. Resveratrol alleviates chronic “real-world” ambient particulate matter-induced lung inflammation and fibrosis by inhibiting NLRP3 inflammasome activation in mice. Ecotoxicol. Environ. Saf. 2019, 182, 109425. [Google Scholar] [CrossRef]
- Wang, J.; He, F.; Chen, L.; Li, Q.; Jin, S.; Zheng, H.; Lin, J.; Zhang, H.; Ma, S.; Mei, J.; et al. Resveratrol inhibits pulmonary fibrosis by regulating miR-21 through MAPK/AP-1 pathways. Biomed. Pharmacother. 2018, 105, 37–44. [Google Scholar] [CrossRef]
- Lee, J.C.; Krochak, R.; Blouin, A.; Kanterakis, S.; Chatterjee, S.; Arguiri, E.; Vachani, A.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol. Ther. 2009, 8, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Christofidou-Solomidou, M.; Tyagi, S.; Tan, K.S.; Hagan, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Heitjan, D.F.; Solomides, C.C.; Cengel, K.A. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer 2011, 11, 269. [Google Scholar] [CrossRef] [Green Version]
- Pietrofesa, R.; Turowski, J.; Tyagi, S.; Dukes, F.; Arguiri, E.; Busch, T.M.; Gallagher-Colombo, S.M.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer 2013, 13, 179. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Dong, X.; Zhao, N.; Su, X.; Li, Y.; Wen, M.; Li, Z.; Wang, C.; Chen, J.; Zhuang, W. Schisandrin B attenuates bleomycin-induced pulmonary fibrosis in mice through the wingless/integrase-1 signaling pathway. Exp. Lung Res. 2020, 46, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, B.; Cao, B.; Wei, F.; Yu, X.; Li, G.F.; Chen, H.; Wei, L.Q.; Wang, P.L. Synergistic protection of Schizandrin B and Glycyrrhizic acid against bleomycin-induced pulmonary fibrosis by inhibiting TGF-β1/Smad2 pathways and overexpression of NOX4. Int. Immunopharmacol. 2017, 48, 67–75. [Google Scholar] [CrossRef]
- Pae, M.; Wu, D. Immunomodulating effects of epigallocatechin-3-gallate from green tea: Mechanisms and applications. Food Funct. 2013, 4, 1287–1303. [Google Scholar] [CrossRef] [PubMed]
- Donà, M.; Dell’Aica, I.; Calabrese, F.; Benelli, R.; Morini, M.; Albini, A.; Garbisa, S. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J. Immunol. 2003, 170, 4335–4341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriram, N.; Kalayarasan, S.; Manikandan, R.; Arumugam, M.; Sudhandiran, G. Epigallocatechin gallate attenuates fibroblast proliferation and excessive collagen production by effectively intervening TGF-β1 signalling. Clin. Exp. Pharmacol. Physiol. 2015, 42, 849–859. [Google Scholar] [CrossRef]
- Li, C.; Sun, X.; Li, A.; Mo, M.; Zhao, Z. S-Allylmercaptocysteine attenuates Bleomycin-induced pulmonary fibrosis in mice via suppressing TGF-β1/Smad and oxidative stress pathways. Int. Immunopharmacol. 2020, 79, 106110. [Google Scholar] [CrossRef]
- Nie, Y.; Yu, K.; Li, B.; Hu, Y.; Zhang, H.; Xin, R.; Xiong, Y.; Zhao, P.; Chai, G. S-allyl-l-cysteine attenuates bleomycin-induced pulmonary fibrosis and inflammation via AKT/NF-κB signaling pathway in mice. J. Pharmacol. Sci. 2019, 139, 377–384. [Google Scholar] [CrossRef]
- Mizuguchi, S.; Takemura, S.; Minamiyama, Y.; Kodai, S.; Tsukioka, T.; Inoue, K.; Okada, S.; Suehiro, S. S-allyl cysteine attenuated CCl4-induced oxidative stress and pulmonary fibrosis in rats. Biofactors 2006, 26, 81–92. [Google Scholar] [CrossRef]
- Moustafa, G.G.; Hussein, M.M.A. New insight on using aged garlic extract against toxic impacts of titanium dioxide bulk salt triggers inflammatory and fibrotic cascades in male rats. Biomed. Pharmacother. 2016, 84, 687–697. [Google Scholar] [CrossRef]
- Tsukioka, T.; Takemura, S.; Minamiyama, Y.; Mizuguchi, S.; Toda, M.; Okada, S. Attenuation of Bleomycin-Induced Pulmonary Fibrosis in Rats with S-Allyl Cysteine. Molecules 2017, 22, 543. [Google Scholar] [CrossRef]
- Oka, V.O.; Okon, U.E.; Osim, E.E. Pulmonary Responses Following Quercetin Administration in Rats After Intratracheal Instillation of Amiodarone. Niger. J. Physiol. Sci. 2019, 34, 63–68. [Google Scholar] [PubMed]
- Verma, R.; Kushwah, L.; Gohel, D.; Patel, M.; Marvania, T.; Balakrishnan, S. Evaluating the Ameliorative Potential of Quercetin against the Bleomycin-Induced Pulmonary Fibrosis in Wistar Rats. Pulm. Med. 2013, 2013, 921724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Impellizzeri, D.; Talero, E.; Siracusa, R.; Alcaide, A.; Cordaro, M.; Maria Zubelia, J.; Bruschetta, G.; Crupi, R.; Esposito, E.; Cuzzocrea, S.; et al. Protective effect of polyphenols in an inflammatory process associated with experimental pulmonary fibrosis in mice. Br. J. Nutr. 2015, 114, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Punithavathi, D.; Venkatesan, N.; Babu, M. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br. J. Pharmacol. 2000, 131, 169–172. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Nazari, Z.; Samei, M. A comparative study of grape seed extract and vitamin E effects on silica-induced pulmonary fibrosis in rats. Pulm. Pharmacol. Ther. 2008, 21, 668–674. [Google Scholar] [CrossRef]
- Song, X.; Wang, B.; Lin, S.; Jing, L.; Mao, C.; Xu, P.; Lv, C.; Liu, W.; Zuo, J. Astaxanthin inhibits apoptosis in alveolar epithelial cells type II in vivo and in vitro through the ROS-dependent mitochondrial signalling pathway. J. Cell Mol. Med. 2014, 18, 2198–2212. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Song, X.; Liu, W.; Zhang, L.; Wang, X.; Lv, C. Astaxanthin ameliorates lung fibrosis in vivo and in vitro by preventing transdifferentiation, inhibiting proliferation, and promoting apoptosis of activated cells. Food Chem. Toxicol. 2013, 56, 450–458. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, P.; Wang, Y.; Wang, M.; Li, H.; Lin, S.; Mao, C.; Wang, B.; Song, X.; Lv, C. Astaxanthin prevents pulmonary fibrosis by promoting myofibroblast apoptosis dependent on Drp1-mediated mitochondrial fission. J. Cell Mol. Med. 2015, 19, 2215–2231. [Google Scholar] [CrossRef] [Green Version]
- Mehrabani, M.; Goudarzi, M.; Mehrzadi, S.; Siahpoosh, A.; Mohammadi, M.; Khalili, H.; Malayeri, A. Crocin: A protective natural antioxidant against pulmonary fibrosis induced by bleomycin. Pharmacol. Rep. 2020. [Google Scholar] [CrossRef]
- Zaghloul, M.S.; Said, E.; Suddek, G.M.; Salem, H.A. Crocin attenuates lung inflammation and pulmonary vascular dysfunction in a rat model of bleomycin-induced pulmonary fibrosis. Life Sci. 2019, 235, 116794. [Google Scholar] [CrossRef]
- Zhou, C.; Han, W.; Zhang, P.; Cai, M.; Wei, D.; Zhang, C. Lycopene from tomatoes partially alleviates the bleomycin-induced experimental pulmonary fibrosis in rats. Nutr. Res. 2008, 28, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Gungor, H.; Ekici, M.; Onder Karayigit, M.; Turgut, N.H.; Kara, H.; Arslanbas, E. Zingerone ameliorates oxidative stress and inflammation in bleomycin-induced pulmonary fibrosis: Modulation of the expression of TGF-β1 and iNOS. Naunyn Schmiedebergs Arch. Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Saba; Khan, S.; Parvez, S.; Chaudhari, B.; Ahmad, F.; Anjum, S.; Raisuddin, S. Ellagic acid attenuates bleomycin and cyclophosphamide-induced pulmonary toxicity in Wistar rats. Food Chem. Toxicol. 2013, 58, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Agackiran, Y.; Gul, H.; Gunay, E.; Akyurek, N.; Memis, L.; Gunay, S.; Sirin, Y.S.; Ide, T. The efficiency of proanthocyanidin in an experimental pulmonary fibrosis model: Comparison with taurine. Inflammation 2012, 35, 1402–1410. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [Green Version]
- Dickson, R.P.; Harari, S.; Kolb, M. Making the case for causality: What role do lung microbiota play in idiopathic pulmonary fibrosis? Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef] [Green Version]
- Dickson, R.P.; Huffnagle, G.B. The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease. PLoS Pathog 2015, 11, e1004923. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef] [Green Version]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef]
- Zhang, D.; Li, S.; Wang, N.; Tan, H.Y.; Zhang, Z.; Feng, Y. The Cross-Talk Between Gut Microbiota and Lungs in Common Lung Diseases. Front. Microbiol. 2020, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, R.; Barnett, J.; Rawal, B.; Nair, A.; Ghai, P.; Kingston, S.; Chua, F.; Wu, Z.; Wells, A.U.; Renzoni, E.R.; et al. Bacterial burden in the lower airways predicts disease progression in idiopathic pulmonary fibrosis and is independent of radiological disease extent. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, X.; Wang, J.; Lou, Q.; Lou, Y.; Li, L.; Wang, H.; Chen, J.; Wu, M.; Song, X.; et al. Dysregulated Lung Commensal Bacteria Drive Interleukin-17B Production to Promote Pulmonary Fibrosis through Their Outer Membrane Vesicles. Immunity 2019, 50, 692–706.e697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, M.K.; Zhou, Y.; Murray, S.; Tayob, N.; Noth, I.; Lama, V.N.; Moore, B.B.; White, E.S.; Flaherty, K.R.; Huffnagle, G.B.; et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: An analysis of the COMET study. Lancet Respir. Med. 2014, 2, 548–556. [Google Scholar] [CrossRef] [Green Version]
- Dickson, R.P.; Huffnagle, G.B.; Flaherty, K.R.; White, E.S.; Martinez, F.J.; Erb-Downward, J.R.; Moore, B.B.; O’Dwyer, D.N. Radiographic Honeycombing and Altered Lung Microbiota in Patients with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Anstrom, K.J.; Noth, I.; Flaherty, K.R.; Edwards, R.H.; Albright, J.; Baucom, A.; Brooks, M.; Clark, A.B.; Clausen, E.S.; Durheim, M.T.; et al. Design and rationale of a multi-center, pragmatic, open-label randomized trial of antimicrobial therapy—The study of clinical efficacy of antimicrobial therapy strategy using pragmatic design in Idiopathic Pulmonary Fibrosis (CleanUP-IPF) clinical trial. Respir. Res. 2020, 21, 68. [Google Scholar] [CrossRef]
- Hammond, M.; Clark, A.B.; Cahn, A.P.; Chilvers, E.R.; Fraser, W.D.; Livermore, D.M.; Maher, T.M.; Parfrey, H.; Swart, A.M.; Stirling, S.; et al. The Efficacy and Mechanism Evaluation of Treating Idiopathic Pulmonary fibrosis with the Addition of Co-trimoxazole (EME-TIPAC): Study protocol for a randomised controlled trial. Trials 2018, 19, 89. [Google Scholar] [CrossRef] [Green Version]
- Madan, J.C.; Koestler, D.C.; Stanton, B.A.; Davidson, L.; Moulton, L.A.; Housman, M.L.; Moore, J.H.; Guill, M.F.; Morrison, H.G.; Sogin, M.L.; et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: Interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Yang, Z.; Zhang, X.; Han, N.; Yuan, J.; Cheng, Y. 16S rDNA analysis of the effect of fecal microbiota transplantation on pulmonary and intestinal flora. 3 Biotech. 2017, 7, 370. [Google Scholar] [CrossRef]
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012, 5, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molyneaux, P.L.; Mallia, P.; Cox, M.J.; Footitt, J.; Willis-Owen, S.A.; Homola, D.; Trujillo-Torralbo, M.B.; Elkin, S.; Kon, O.M.; Cookson, W.O.; et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1224–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAleer, J.P.; Kolls, J.K. Contributions of the intestinal microbiome in lung immunity. Eur. J. Immunol. 2018, 48, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, S.F.; Espindola, M.S.; Vij, R.; Oldham, J.M.; Huffnagle, G.B.; Erb-Downward, J.R.; Flaherty, K.R.; Moore, B.B.; White, E.S.; et al. Microbes Are Associated with Host Innate Immune Response in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 196, 208–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaplin, A.; Carpéné, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef] [Green Version]
- Ly, N.P.; Litonjua, A.; Gold, D.R.; Celedón, J.C. Gut microbiota, probiotics, and vitamin D: Interrelated exposures influencing allergy, asthma, and obesity? J. Allergy. Clin. Immunol. 2011, 127, 1087–1094. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Rodriguez, J.; Olivares, M.; Neyrinck, A.M. Microbiome response to diet: Focus on obesity and related diseases. Rev. Endocr. Metab Dis. 2020. [Google Scholar] [CrossRef]
- Li, L.; Krause, L.; Somerset, S. Associations between micronutrient intakes and gut microbiota in a group of adults with cystic fibrosis. Clin Nutr. 2017, 36, 1097–1104. [Google Scholar] [CrossRef]
- Li, L.; Somerset, S. Associations between Flavonoid Intakes and Gut Microbiota in a Group of Adults with Cystic Fibrosis. Nutrients 2018, 10, 1264. [Google Scholar] [CrossRef] [Green Version]
- Bernard, H.; Desseyn, J.L.; Bartke, N.; Kleinjans, L.; Stahl, B.; Belzer, C.; Knol, J.; Gottrand, F.; Husson, M.O. Dietary pectin-derived acidic oligosaccharides improve the pulmonary bacterial clearance of Pseudomonas aeruginosa lung infection in mice by modulating intestinal microbiota and immunity. J. Infect. Dis. 2015, 211, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Fabbrizzi, A.; Amedei, A.; Lavorini, F.; Renda, T.; Fontana, G. The lung microbiome: Clinical and therapeutic implications. Intern. Emerg. Med. 2019, 14, 1241–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Lee, H.S.; Hua, H.S.; Wang, C.H.; Yu, M.C.; Chen, B.C.; Lin, C.H. induces connective tissue growth factor expression through the TLR2-JNK-AP-1 pathway in human lung fibroblasts. FASEB J. 2019, 33, 12554–12564. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.K.; Kim, R.Y.; Karim, R.; Mayall, J.R.; Martin, K.L.; Shahandeh, A.; Abbasian, F.; Starkey, M.R.; Loustaud-Ratti, V.; Johnstone, D.; et al. Role of iron in the pathogenesis of respiratory disease. Int. J. Biochem. Cell. Biol. 2017, 88, 181–195. [Google Scholar] [CrossRef]
- Scoditti, E.; Massaro, M.; Garbarino, S.; Toraldo, D.M. Role of Diet in Chronic Obstructive Pulmonary Disease Prevention and Treatment. Nutrients 2019, 11, 1357. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercader-Barceló, J.; Truyols-Vives, J.; Río, C.; López-Safont, N.; Sala-Llinàs, E.; Chaplin, A. Insights into the Role of Bioactive Food Ingredients and the Microbiome in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 6051. https://doi.org/10.3390/ijms21176051
Mercader-Barceló J, Truyols-Vives J, Río C, López-Safont N, Sala-Llinàs E, Chaplin A. Insights into the Role of Bioactive Food Ingredients and the Microbiome in Idiopathic Pulmonary Fibrosis. International Journal of Molecular Sciences. 2020; 21(17):6051. https://doi.org/10.3390/ijms21176051
Chicago/Turabian StyleMercader-Barceló, Josep, Joan Truyols-Vives, Carlos Río, Nora López-Safont, Ernest Sala-Llinàs, and Alice Chaplin. 2020. "Insights into the Role of Bioactive Food Ingredients and the Microbiome in Idiopathic Pulmonary Fibrosis" International Journal of Molecular Sciences 21, no. 17: 6051. https://doi.org/10.3390/ijms21176051
APA StyleMercader-Barceló, J., Truyols-Vives, J., Río, C., López-Safont, N., Sala-Llinàs, E., & Chaplin, A. (2020). Insights into the Role of Bioactive Food Ingredients and the Microbiome in Idiopathic Pulmonary Fibrosis. International Journal of Molecular Sciences, 21(17), 6051. https://doi.org/10.3390/ijms21176051