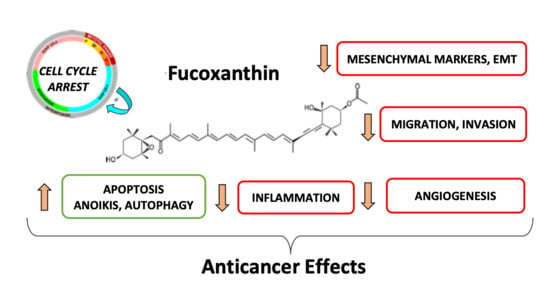
Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy
Abstract
:1. Introduction
2. Absorption and Metabolites of Fucoxanthin
3. Antiproliferative Effects of Fucoxanthin through Cell Cycle Arrest in Cancer Cells
4. Induction of Apoptosis and Autophagy in Cancer Cells
4.1. Apoptosis
4.2. Anoikis
4.3. Autophagy
5. Involvement in DNA Damages
6. Inhibition of Metastasis-Related Migration, Invasion and Epithelial–Mesenchymal Transition
7. Anti-Angiogenic Effect of Fucoxanthin
8. Anti-Inflammatory Effects of Fucoxanthin
9. Fucoxanthin in Clinical Trials
10. Discussion
11. Conclusions
Authors Contributions
Funding
Conflicts of Interest
Abbreviations
| AP-1 | activator protein-1 |
| ASC | apoptosis-associated speck-like protein |
| ATM | ataxia telangiectasia mutated protein |
| ATR | ataxia telangiectasia and Rad3-related protein |
| BMI | body mass index |
| CAT | catalase |
| CDK | cyclin-dependent kinase |
| CXCR4 | C-X-C motif chemokine receptor 4 |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial–mesenchymal transition |
| ERCC1 | excision repair cross complementation 1 |
| ERK | extracellular signal-regulated kinase |
| FGF | fibroblast growth factor |
| HIF-1 | hypoxia-inducible factor-1 |
| HLEC | human lymphatic endothelial cells |
| HTLV-1 | human T-cell leukemia virus type 1 |
| HUVEC | human umbilical vein endothelial cells |
| IL | Interleukin |
| JNK | Jun-N-terminal kinase |
| LC3 | microtubule-associated protein 1A/1B-light chain 3 |
| LPS | lipopolysaccharide |
| MAPK | mitogen activated kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| MMP | matrix metalloproteinase |
| mTOR | mechanistic target of rapamycin |
| NAC | N-acetyl cysteine |
| NER | nucleotide excision repair |
| NFE2L2/Nrf2 | nuclear factor (erythroid-derived) like-2 |
| NF-κB | nuclear factor κB |
| NLRP3 | NOD-like receptor family, pyrin domain containing 3 |
| PARP | poly-ADP-ribose polymerase |
| PI3K | phosphoinositide-3 kinase |
| p-FAK | phosphorylated focal adhesion kinase |
| PGI2 | prostacyclin |
| pRb | retinoblastoma protein |
| PPAR | peroxysome proliferator-activated receptor |
| PTEN | phosphatase and tensin homolog protein |
| PUMA | p53 upregulated modulator of apoptosis |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| STAT | signal transducer and activator of transcription 3 |
| TNFα | tumor necrosis factor α |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
| UCP1 | uncoupling protein-1 |
| VEGF | vascular endothelial growth factor |
| XIAP | X-linked inhibitor of apoptosis |
References
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, G.A.; Hearst, J.E. Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 1996, 10, 228–237. [Google Scholar] [CrossRef]
- Tinkler, J.H.; Böhm, F.; Schalch, W.; Truscott, T.G. Dietary carotenoids protect human cells from damage. J. Photochem. Photobiol. B 1994, 26, 283–285. [Google Scholar] [CrossRef]
- Foote, C.S.; Denny, R.W. Chemistry of singlet oxygen. VII. Quenching by .beta.-carotene. J. Am. Chem. Soc. 1968, 90, 6233–6235. [Google Scholar] [CrossRef]
- Jørgensen, K.; Skibsted, L.H. Carotenoid scavenging of radicals. Effect of carotenoid structure and oxygen partial pressure on antioxidative activity. Z. Lebensm. Unters. Forsch. 1993, 196, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Willstätter, R.; Page, H.J. Untersuchungen über Chlorophyll. XXIV. Über die Pigmente der Braunalgen. Justus Liebigs Ann. Chem. 1914, 404, 237–271. [Google Scholar] [CrossRef] [Green Version]
- Bonnett, R.; Mallams, A.K.; Tee, J.L.; Weedon, B.C.L.; McCormick, A. Fucoxanthin and related pigments. Chem. Commun. Lond. 1966, 515. [Google Scholar] [CrossRef]
- Bonnett, R.; Mallams, A.K.; Spark, A.A.; Tee, J.L.; Weedon, B.C.L.; McCormick, A. Carotenoids and related compounds. Part XX. Structure and reactions of fucoxanthin. J. Chem. Soc. C Org. 1969, 429. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Liaaen-Jensen, S. Marine Carotenoids. In Marine Natural Products; Elsevier: Amsterdam, The Netherlands, 1978; pp. 1–73. ISBN 978-0-12-624002-3. [Google Scholar]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary Combination of Fucoxanthin and Fish Oil Attenuates the Weight Gain of White Adipose Tissue and Decreases Blood Glucose in Obese/Diabetic KK- A y Mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol. Cell. Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin and Rosmarinic Acid Combination Has Anti-Inflammatory Effects through Regulation of NLRP3 Inflammasome in UVB-Exposed HaCaT Keratinocytes. Mar. Drugs 2019, 17, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, P.-A.; Phan, N.N.; Lu, W.-J.; Ngoc Hieu, B.T.; Lin, Y.-C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, C.; Côme, M.; Ulmann, L.; Chini Zittelli, G.; Faraloni, C.; Nazih, H.; Ouguerram, K.; Chénais, B.; Mimouni, V. Preventive Effects of the Marine Microalga Phaeodactylum tricornutum, Used as a Food Supplement, on Risk Factors Associated with Metabolic Syndrome in Wistar Rats. Nutrients 2019, 11, 1069. [Google Scholar] [CrossRef] [Green Version]
- Airanthi, M.K.W.-A.; Hosokawa, M.; Miyashita, K. Comparative Antioxidant Activity of Edible Japanese Brown Seaweeds. J. Food Sci. 2011, 76, C104–C111. [Google Scholar] [CrossRef]
- Tafuku, S.; Ishikawa, C.; Yasumoto, T.; Mori, N. Anti-neoplastic effects of fucoxanthin and its deacetylated product, fucoxanthinol, on Burkitt’s and Hodgkin’s lymphoma cells. Oncol. Rep. 2012, 28, 1512–1518. [Google Scholar] [CrossRef]
- Rokkaku, T.; Kimura, R.; Ishikawa, C.; Yasumoto, T.; Senba, M.; Kanaya, F.; Mori, N. Anticancer effects of marine carotenoids, fucoxanthin and its deacetylated product, fucoxanthinol, on osteosarcoma. Int. J. Oncol. 2013, 43, 1176–1186. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Ahn, G.-N.; Kang, S.-M.; Kang, D.-H.; Affan, A.; Oh, C.; Jung, W.-K.; Jeon, Y.-J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Imbs, T.I.; Ermakova, S.P.; Fedoreyev, S.A.; Anastyuk, S.D.; Zvyagintseva, T.N. Isolation of Fucoxanthin and Highly Unsaturated Monogalactosyldiacylglycerol from Brown Alga Fucus evanescens C Agardh and In Vitro Investigation of Their Antitumor Activity. Mar. Biotechnol. 2013, 15, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Zaragozá, M.C.; López, D.; Sáiz, P.M.; Poquet, M.; Pérez, J.; Puig-Parellada, P.; Màrmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and Antioxidant Activity in Vitro and in Vivo of Two Fucus vesiculosus Extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Heo, S.-J.; Kang, S.-M.; Ahn, G.; Jeon, Y.-J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. In Vitro 2010, 24, 1648–1654. [Google Scholar] [CrossRef]
- Kanazawa, K.; Ozaki, Y.; Hashimoto, T.; Das, S.K.; Matsushita, S.; Hirano, M.; Okada, T.; Komoto, A.; Mori, N.; Nakatsuka, M. Commercial-scale Preparation of Biofunctional Fucoxanthin from Waste Parts of Brown Sea Algae Laminalia japonica. Food Sci. Technol. Res. 2008, 14, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography: Other Techniques. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef]
- Sangeetha, R.K.; Bhaskar, N.; Divakar, S.; Baskaran, V. Bioavailability and metabolism of fucoxanthin in rats: Structural characterization of metabolites by LC-MS (APCI). Mol. Cell. Biochem. 2010, 333, 299–310. [Google Scholar] [CrossRef]
- Mori, K.; Ooi, T.; Hiraoka, M.; Oka, N.; Hamada, H.; Tamura, M.; Kusumi, T. Fucoxanthin and Its Metabolites in Edible Brown Algae Cultivated in Deep Seawater. Mar. Drugs 2004, 2, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Chung, T.-W.; Choi, H.-J.; Lee, J.-Y.; Jeong, H.-S.; Kim, C.-H.; Joo, M.; Choi, J.-Y.; Han, C.-W.; Kim, S.-Y.; Choi, J.-S.; et al. Marine algal fucoxanthin inhibits the metastatic potential of cancer cells. Biochem. Biophys. Res. Commun. 2013, 439, 580–585. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Miyashita, K. Fucoxanthin Extractions of Brown Seaweeds and Analysis of Their Lipid Fraction in Methanol. Food Sci. Technol. Res. 2012, 18, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Nagappan, H.; Pee, P.P.; Kee, S.H.Y.; Ow, J.T.; Yan, S.W.; Chew, L.Y.; Kong, K.W. Malaysian brown seaweeds Sargassum siliquosum and Sargassum polycystum: Low density lipoprotein (LDL) oxidation, angiotensin converting enzyme (ACE), α-amylase, and α-glucosidase inhibition activities. Food Res. Int. Ott. Ont. 2017, 99, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Oh, C.; Choi, Y.-U.; Yoon, K.-T.; Kang, D.-H.; Qian, Z.-J.; Choi, I.-W.; Jung, W.-K. Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Fukuda, S.; Izumi, H.; Saga, N. Anti-Oxidant and Fucoxanthin Contents of Brown Alga Ishimozuku (Sphaerotrichia divaricata) from the West Coast of Aomori, Japan. Mar. Drugs 2018, 16, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurrochmad, A.; Wirasti, W.; Dirman, A.; Lukitaningsih, E.; Rahmawati, A.; Fakhrudin, N. Effects of Antioxidant, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase of The Extract and Fraction From Turbinaria decurrens Bory. Indones. J. Pharm. 2018, 29, 188. [Google Scholar] [CrossRef]
- Petrushkina, M.; Gusev, E.; Sorokin, B.; Zotko, N.; Mamaeva, A.; Filimonova, A.; Kulikovskiy, M.; Maltsev, Y.; Yampolsky, I.; Guglya, E.; et al. Fucoxanthin production by heterokont microalgae. Algal Res. 2017, 24, 387–393. [Google Scholar] [CrossRef]
- Allen, M.B.; Goodwin, T.W.; Phagpolngarm, S. Carotenoid Distribution in Certain Naturally Occurring Algae and in some Artificially Induced Mutants of Chlorella pyrenoidosa. J. Gen. Microbiol. 1960, 23, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Withers, N.W.; Fiksdahl, A.; Tuttle, R.C.; Liaaen-Jensen, S. Carotenoids of the chrysophyceae. Comp. Biochem. Physiol. Part. B Comp. Biochem. 1981, 68, 345–349. [Google Scholar] [CrossRef]
- Foo, S.C.; Md. Yusoff, F.; Ismail, M.; Basri, M.; Chan, K.W.; Khong, N.M.H.; Yau, S.K. Production of fucoxanthin-rich fraction (FxRF) from a diatom, Chaetoceros calcitrans (Paulsen) Takano 1968. Algal Res. 2015, 12, 26–32. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, Characterization, and Antioxidant Activity of Fucoxanthin from the Marine Diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.-L.; Wang, G.; Chen, L.; et al. Fucoxanthin, a Marine Xanthophyll Isolated From Conticribra weissflogii ND-8: Preventive Anti-Inflammatory Effect in a Mouse Model of Sepsis. Front. Pharmacol. 2019, 10, 906. [Google Scholar] [CrossRef] [Green Version]
- Crupi, P.; Toci, A.T.; Mangini, S.; Wrubl, F.; Rodolfi, L.; Tredici, M.R.; Coletta, A.; Antonacci, D. Determination of fucoxanthin isomers in microalgae (Isochrysis sp.) by high-performance liquid chromatography coupled with diode-array detector multistage mass spectrometry coupled with positive electrospray ionization: Fucoxanthin isomers in Isochrysis sp. Rapid Commun. Mass Spectrom. 2013, 27, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.-M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process. Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown Algae Fucoxanthin Is Hydrolyzed to Fucoxanthinol during Absorption by Caco-2 Human Intestinal Cells and Mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of Fucoxanthinol into Amarouciaxanthin a in mice and HepG2 cells: Formation and cytotoxicity of Fucoxanthin metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komba, S.; Kotake-Nara, E.; Tsuzuki, W. Degradation of Fucoxanthin to Elucidate the Relationship between the Fucoxanthin Molecular Structure and Its Antiproliferative Effect on Caco-2 Cells. Mar. Drugs 2018, 16, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, Y.; Ishibashi, M.; Yamaguchi, N.; Kobayashi, J. Isolation of Apo-9’-fucoxanthinone from the Cultured Marine Dinoflagellate Amphidinium sp. J. Nat. Prod. 1995, 58, 1097–1099. [Google Scholar] [CrossRef]
- Shaw, B.A.; Andersen, R.J.; Harrison, P.J. Feeding deterrence properties of apo-fucoxanthinoids from marine diatoms. I. Chemical structures of apo-fucoxanthinoids produced by Phaeodactylum tricornutum. Mar. Biol. 1995, 124, 467–472. [Google Scholar] [CrossRef]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Almeida, T.P.; Ferreira, J.; Vettorazzi, A.; Azqueta, A.; Rocha, E.; Ramos, A.A. Cytotoxic activity of fucoxanthin, alone and in combination with the cancer drugs imatinib and doxorubicin, in CML cell lines. Environ. Toxicol. Pharmacol. 2018, 59, 24–33. [Google Scholar] [CrossRef]
- Ishikawa, C.; Tafuku, S.; Kadekaru, T.; Sawada, S.; Tomita, M.; Okudaira, T.; Nakazato, T.; Toda, T.; Uchihara, J.-N.; Taira, N.; et al. Anti-adult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. Int. J. Cancer 2008, 123, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ishikawa, C.; Katano, H.; Yasumoto, T.; Mori, N. Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Lett. 2011, 300, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zeng, Y.; Liu, Y.; Hu, X.; Li, S.; Wang, Y.; Li, L.; Lei, Z.; Zhang, Z. Fucoxanthin induces growth arrest and apoptosis in human bladder cancer T24 cells by up-regulation of p21 and down-regulation of mortalin. Acta Biochim. Biophys. Sin. 2014, 46, 877–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-N.; Ahn, G.; Heo, S.-J.; Kang, S.-M.; Kang, M.-C.; Yang, H.-M.; Kim, D.; Roh, S.W.; Kim, S.-K.; Jeon, B.-T.; et al. Inhibition of tumor growth in vitro and in vivo by fucoxanthin against melanoma B16F10 cells. Environ. Toxicol. Pharmacol. 2013, 35, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.-X.; Hu, X.-M.; Xu, S.-Q.; Jiang, Z.-J.; Yang, W. Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway. Eur. J. Pharmacol. 2011, 657, 10–19. [Google Scholar] [CrossRef]
- Das, S.K.; Hashimoto, T.; Shimizu, K.; Yoshida, T.; Sakai, T.; Sowa, Y.; Komoto, A.; Kanazawa, K. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim. Biophys. Acta BBA Gen. Subj. 2005, 1726, 328–335. [Google Scholar] [CrossRef]
- Satomi, Y. Fucoxanthin induces GADD45A expression and G1 arrest with SAPK/JNK activation in LNCap human prostate cancer cells. Anticancer Res. 2012, 32, 807–813. [Google Scholar]
- Satomi, Y.; Nishino, H. Implication of mitogen-activated protein kinase in the induction of G1 cell cycle arrest and gadd45 expression by the carotenoid fucoxanthin in human cancer cells. Biochim. Biophys. Acta 2009, 1790, 260–266. [Google Scholar] [CrossRef]
- Yoshiko, S.; Hoyoku, N. Fucoxanthin, a natural carotenoid, induces G1 arrest and GADD45 gene expression in human cancer cells. In Vivo Athens Greece 2007, 21, 305–309. [Google Scholar]
- Okuzumi, J.; Nishino, H.; Murakoshi, M.; Iwashima, A.; Tanaka, Y.; Yamane, T.; Fujita, Y.; Takahashi, T. Inhibitory effects of fucoxanthin, a natural carotenoid, on N-myc expression and cell cycle progression in human malignant tumor cells. Cancer Lett. 1990, 55, 75–81. [Google Scholar] [CrossRef]
- Das, S.K.; Hashimoto, T.; Kanazawa, K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. Biochim. Biophys. Acta 2008, 1780, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S. Fucoxanthin, A Carotenoid Derived from Phaeodactylum tricornutum Exerts Antiproliferative and Antioxidant Activities in Vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-L.; Huang, Y.-S.; Hosokawa, M.; Miyashita, K.; Hu, M.-L. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication. Chem. Biol. Interact. 2009, 182, 165–172. [Google Scholar] [CrossRef]
- Hou, L.; Gao, C.; Chen, L.; Hu, G.; Xie, S. Essential role of autophagy in fucoxanthin-induced cytotoxicity to human epithelial cervical cancer HeLa cells. Acta Pharmacol. Sin. 2013, 34, 1403–1410. [Google Scholar] [CrossRef] [Green Version]
- Moreau, D.; Tomasoni, C.; Jacquot, C.; Kaas, R.; Le Guedes, R.; Cadoret, J.-P.; Muller-Feuga, A.; Kontiza, I.; Vagias, C.; Roussis, V.; et al. Cultivated microalgae and the carotenoid fucoxanthin from Odontella aurita as potent anti-proliferative agents in bronchopulmonary and epithelial cell lines. Environ. Toxicol. Pharmacol. 2006, 22, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Zhou, S.; Zhu, L.; Ming, J.; Zeng, F.; Xu, R. Antitumor Effects of Laminaria Extract Fucoxanthin on Lung Cancer. Mar. Drugs 2017, 15, 39. [Google Scholar] [CrossRef]
- Liu, C.-L.; Lim, Y.-P.; Hu, M.-L. Fucoxanthin enhances cisplatin-induced cytotoxicity via NFκB-mediated pathway and downregulates DNA repair gene expression in human hepatoma HepG2 cells. Mar. Drugs 2013, 11, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, S.C.; Md. Yusoff, F.; Imam, M.U.; Foo, J.B.; Ismail, N.; Azmi, N.H.; Tor, Y.S.; Khong, N.M.H.; Ismail, M. Increased fucoxanthin in Chaetoceros calcitrans extract exacerbates apoptosis in liver cancer cells via multiple targeted cellular pathways. Biotechnol. Rep. 2019, 21, e00296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cheng, J.; Min, Z.; Yin, T.; Zhang, R.; Zhang, W.; Hu, L.; Cui, Z.; Gao, C.; Xu, S.; et al. Effects of fucoxanthin on autophagy and apoptosis in SGC-7901cells and the mechanism. J. Cell. Biochem. 2018, 119, 7274–7284. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine Carotenoid Fucoxanthin Possesses Anti-Metastasis Activity: Molecular Evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, M.; Kudo, M.; Maeda, H.; Kohno, H.; Tanaka, T.; Miyashita, K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta BBA Gen. Subj. 2004, 1675, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Costa, E.; Abreu, M.; Gargiulo, D.; Rocha, E.; Ramos, A.A. Anticancer effects of seaweed compounds fucoxanthin and phloroglucinol, alone and in combination with 5-fluorouracil in colon cells. J. Toxicol. Environ. Health A 2017, 80, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Konishi, I.; Hosokawa, M.; Sashima, T.; Kobayashi, H.; Miyashita, K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2006, 142, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, P.; Hamada, M.; Takahashi, S.; Xing, G.; Liu, J.; Sugiura, N. Potential chemoprevention effect of dietary fucoxanthin on urinary bladder cancer EJ-1 cell line. Oncol. Rep. 2008, 20, 1099–1103. [Google Scholar] [CrossRef] [Green Version]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids Affect Proliferation of Human Prostate Cancer Cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Sugawara, T.; Nagao, A. Antiproliferative effect of neoxanthin and fucoxanthin on cultured cells. Fish. Sci. 2005, 71, 459–461. [Google Scholar] [CrossRef]
- Rwigemera, A.; Mamelona, J.; Martin, L.J. Comparative Effects between Fucoxanthinol and its Precursor Fucoxanthin on Viability and Apoptosis of Breast Cancer Cell Lines MCF-7 and MDA-MB-23. Anticancer Res. 2015, 35, 207–219. [Google Scholar]
- Ayyad, S.-E.; Basaif, S.; Badria, A.; Ezmirly, S.; Alarif, W.; Badria, F. Antioxidant, cytotoxic, antitumor, and protective DNA damage metabolites from the red sea brown alga Sargassum sp. Pharmacogn. Res. 2011, 3, 160. [Google Scholar] [CrossRef] [Green Version]
- Rwigemera, A.; Mamelona, J.; Martin, L.J. Inhibitory effects of fucoxanthinol on the viability of human breast cancer cell lines MCF-7 and MDA-MB-231 are correlated with modulation of the NF-kappaB pathway. Cell Biol. Toxicol. 2014, 30, 157–167. [Google Scholar] [CrossRef]
- Ye, G.; Lu, Q.; Zhao, W.; Du, D.; Jin, L.; Liu, Y. Fucoxanthin induces apoptosis in human cervical cancer cell line HeLa via PI3K/Akt pathway. Tumor Biol. 2014, 35, 11261–11267. [Google Scholar] [CrossRef]
- Afzal, S.; Garg, S.; Ishida, Y.; Terao, K.; Kaul, S.; Wadhwa, R. Rat Glioma Cell-Based Functional Characterization of Anti-Stress and Protein Deaggregation Activities in the Marine Carotenoids, Astaxanthin and Fucoxanthin. Mar. Drugs 2019, 17, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Qiu, S.; Shao, N.; Zheng, J. Fucoxanthin and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Synergistically Promotes Apoptosis of Human Cervical Cancer Cells by Targeting PI3K/Akt/NF-κB Signaling Pathway. Med. Sci. Monit. 2018, 24, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fu, X.; Cao, W.; Xiang, W.; Hou, Y.; Ma, J.; Wang, Y.; Fan, C. Induction of Apoptosis in Human Glioma Cells by Fucoxanthin via Triggering of ROS-Mediated Oxidative Damage and Regulation of MAPKs and PI3K–AKT Pathways. J. Agric. Food Chem. 2019, 67, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, J.; Zhang, Y.; Wang, Z.; Yang, Y.; Bai, M.; Dai, Y. Fucoxanthin Activates Apoptosis via Inhibition of PI3K/Akt/mTOR Pathway and Suppresses Invasion and Migration by Restriction of p38-MMP-2/9 Pathway in Human Glioblastoma Cells. Neurochem. Res. 2016, 41, 2728–2751. [Google Scholar] [CrossRef]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta 2011, 1810, 497–503. [Google Scholar] [CrossRef]
- Sugawara, T.; Matsubara, K.; Akagi, R.; Mori, M.; Hirata, T. Antiangiogenic Activity of Brown Algae Fucoxanthin and Its Deacetylated Product, Fucoxanthinol. J. Agric. Food Chem. 2006, 54, 9805–9810. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell. Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef]
- Nishino, H. Cancer prevention by carotenoids. Mutat. Res. 1998, 402, 159–163. [Google Scholar] [CrossRef]
- Okuzumi, J.; Takahashi, T.; Yamane, T.; Kitao, Y.; Inagake, M.; Ohya, K.; Nishino, H.; Tanaka, Y. Inhibitory effects of fucoxanthin, a natural carotenoid, on N-ethyl-N’-nitro-N-nitrosoguanidine-induced mouse duodenal carcinogenesis. Cancer Lett. 1993, 68, 159–168. [Google Scholar] [CrossRef]
- Nishino, H. Cancer chemoprevention by natural carotenoids and their related compounds. J. Cell. Biochem. 1995, 59, 231–235. [Google Scholar] [CrossRef]
- Terasaki, M.; Iida, T.; Kikuchi, F.; Tamura, K.; Endo, T.; Kuramitsu, Y.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Fucoxanthin potentiates anoikis in colon mucosa and prevents carcinogenesis in AOM/DSS model mice. J. Nutr. Biochem. 2019, 64, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis 1998, 19, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chen, S.; Xu, S.; Yu, X.; Ma, D.; Hu, X.; Cao, X. In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar. Drugs 2012, 10, 2055–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, C.; Lock, R.; Gao, S.; Salas, E.; Debnath, J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol. Biol. Cell 2008, 19, 797–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddig, P.J.; Juliano, R.L. Clinging to life: Cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005, 24, 425–439. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Terasaki, M.; Nagao, A. Characterization of Apoptosis Induced by Fucoxanthin in Human Promyelocytic Leukemia Cells. Biosci. Biotechnol. Biochem. 2005, 69, 224–227. [Google Scholar] [CrossRef]
- Tamura, S.; Narita, T.; Fujii, G.; Miyamoto, S.; Hamoya, T.; Kurokawa, Y.; Takahashi, M.; Miki, K.; Matsuzawa, Y.; Komiya, M.; et al. Inhibition of NF-kappaB transcriptional activity enhances fucoxanthinol-induced apoptosis in colorectal cancer cells. Genes Environ. 2019, 41, 1. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, M.; Maeda, H.; Miyashita, K.; Mutoh, M. Induction of Anoikis in Human Colorectal Cancer Cells by Fucoxanthinol. Nutr. Cancer 2017, 69, 1043–1052. [Google Scholar] [CrossRef]
- Taira, J.; Sonamoto, M.; Uehara, M. Dual Biological Functions of a Cytoprotective Effect and Apoptosis Induction by Bioavailable Marine Carotenoid Fucoxanthinol through Modulation of the Nrf2 Activation in RAW264.7 Macrophage Cells. Mar. Drugs 2017, 15, 305. [Google Scholar] [CrossRef] [Green Version]
- Tomicic, M.T.; Kaina, B. Topoisomerase degradation, DSB repair, p53 and IAPs in cancer cell resistance to camptothecin-like topoisomerase I inhibitors. Biochim. Biophys. Acta BBA Rev. Cancer 2013, 1835, 11–27. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Chan, H.-H.; Chen, S.-H.; Sarvagalla, S.; Chen, P.-S.; Coumar, M.S.; Cheng, S.M.; Chang, Y.-C.; Lin, C.-H.; Leung, E.; et al. BIRC5/Survivin is a novel ATG12–ATG5 conjugate interactor and an autophagy-induced DNA damage suppressor in human cancer and mouse embryonic fibroblast cells. Autophagy 2020, 16, 1296–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Wang, M.; Xiang, Y.; Ru, X.; Ren, Y.; Liu, X.; Qiu, L.; Zhang, Y. Nrf1 Is Endowed with a Dominant Tumor-Repressing Effect onto the Wnt/β-Catenin-Dependent and Wnt/β-Catenin-Independent Signaling Networks in the Human Liver Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 5138539. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.-J.; Chu, P.-Y.; Yiang, G.-T.; Wu, M.-Y. The Molecular Mechanism of Epithelial–Mesenchymal Transition for Breast Carcinogenesis. Biomolecules 2019, 9, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkman, J.; Shing, Y. Angiogenesis. J. Biol. Chem. 1992, 267, 10931–10934. [Google Scholar] [PubMed]
- Folkman, J.; Klagsbrun, M. Angiogenic factors. Science 1987, 235, 442–447. [Google Scholar] [CrossRef]
- Tonini, T.; Rossi, F.; Claudio, P.P. Molecular basis of angiogenesis and cancer. Oncogene 2003, 22, 6549–6556. [Google Scholar] [CrossRef] [Green Version]
- Torisu, H.; Ono, M.; Kiryu, H.; Furue, M.; Ohmoto, Y.; Nakayama, J.; Nishioka, Y.; Sone, S.; Kuwano, M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: Possible involvement of TNFalpha and IL-1alpha. Int. J. Cancer 2000, 85, 182–188. [Google Scholar] [CrossRef]
- Coussens, L.M.; Raymond, W.W.; Bergers, G.; Laig-Webster, M.; Behrendtsen, O.; Werb, Z.; Caughey, G.H.; Hanahan, D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999, 13, 1382–1397. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Tinkle, C.L.; Hanahan, D.; Werb, Z. MMP-9 Supplied by Bone Marrow–Derived Cells Contributes to Skin Carcinogenesis. Cell 2000, 103, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.-W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, J.D.; Shoaibi, M.A.; Maestro, R.; Carnero, A.; Hannon, G.J.; Beach, D.H. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 1999, 190, 1375–1382. [Google Scholar] [CrossRef]
- Martins-Green, M.; Boudreau, N.; Bissell, M.J. Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res. 1994, 54, 4334–4341. [Google Scholar]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Giavazzi, R.; Garofalo, A.; Bani, M.R.; Abbate, M.; Ghezzi, P.; Boraschi, D.; Mantovani, A.; Dejana, E. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res. 1990, 50, 4771–4775. [Google Scholar]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [Green Version]
- Vijay, K.; Sowmya, P.R.-R.; Arathi, B.P.; Shilpa, S.; Shwetha, H.J.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Low-dose doxorubicin with carotenoids selectively alters redox status and upregulates oxidative stress-mediated apoptosis in breast cancer cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 118, 675–690. [Google Scholar] [CrossRef]
- Shin, J.; Song, M.-H.; Oh, J.-W.; Keum, Y.-S.; Saini, R.K. Pro-oxidant Actions of Carotenoids in Triggering Apoptosis of Cancer Cells: A Review of Emerging Evidence. Antioxidants 2020, 9, 532. [Google Scholar] [CrossRef]
- Lin, H.-W.; Chen, Y.-C.; Liu, C.-W.; Yang, D.-J.; Chen, S.-Y.; Chang, T.-J.; Chang, Y.-Y. Regulation of virus-induced inflammatory response by Dunaliella salina alga extract in macrophages. Food Chem. Toxicol. 2014, 71, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.C.; Pagès, A.K.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed Secondary Metabolites with Beneficial Health Effects: An Overview of Successes in In Vivo Studies and Clinical Trials. Mar. Drugs 2019, 18, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, A.; Yonekura, L.; Nagao, A. Low bioavailability of dietary epoxyxanthophylls in humans. Br. J. Nutr. 2008, 100, 273–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of XanthigenTM in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, Y.; Mizuno, M.; Yoshida, M.; Nishitani, Y.; Azuma, T.; Komoto, A.; Maoka, T.; Tanino, Y.; Kanazawa, K. Pharmacokinetics of fucoxanthinol in human plasma after the oral administration of kombu extract. Br. J. Nutr. 2012, 107, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Mikami, N.; Hosokawa, M.; Miyashita, K.; Sohma, H.; Ito, Y.M.; Kokai, Y. Reduction of HbA1c levels by fucoxanthin-enriched akamoku oil possibly involves the thrifty allele of uncoupling protein 1 (UCP1): A randomised controlled trial in normal-weight and obese Japanese adults. J. Nutr. Sci. 2017, 6, e5. [Google Scholar] [CrossRef] [Green Version]
- Ren, R.; Azuma, Y.; Ojima, T.; Hashimoto, T.; Mizuno, M.; Nishitani, Y.; Yoshida, M.; Azuma, T.; Kanazawa, K. Modulation of platelet aggregation-related eicosanoid production by dietary F-fucoidan from brown alga Laminaria japonica in human subjects. Br. J. Nutr. 2013, 110, 880–890. [Google Scholar] [CrossRef] [Green Version]
- Hitoe, S.; Shimoda, H. Seaweed Fucoxanthin Supplementation Improves Obesity Parameters in Mild Obese Japanese Subjects. Funct. Foods Health Dis. 2017, 7, 246. [Google Scholar] [CrossRef]
- ClinicalTrials.gov is a Database of Privately and Publicly Funded Clinical Studies Conducted Around the World. It is a resource provided by the United States National Library of Medicine. Available online: https://clinicaltrials.gov/ (accessed on 21 July 2020)(updated on 23 October 2020).
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.; Dutreix, M. DNA repair inhibitors to enhance radiotherapy: Progresses and limitations. Cancer Radiother. J. Soc. Francaise Radiother. Oncol. 2019, 23, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.R.R.; Silva, M.M.; Quinet, A.; Cabral-Neto, J.B.; Menck, C.F.M. DNA repair pathways and cisplatin resistance: An intimate relationship. Clin. Sao Paulo Braz. 2018, 73, e478s. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Rosen, R.; Vagaggini, T.; Chen, Y.; Hu, D.-N. Zeaxanthin inhibits hypoxia-induced VEGF secretion by RPE cells through decreased protein levels of hypoxia-inducible factors-1α. BioMed Res. Int. 2015, 2015, 687386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Kwon, S.-H.; Chun, Y.S.; Gu, M.-Y.; Yang, H.O. Anti-Neuroinflammatory Effects of Fucoxanthin via Inhibition of Akt/NF-κB and MAPKs/AP-1 Pathways and Activation of PKA/CREB Pathway in Lipopolysaccharide-Activated BV-2 Microglial Cells. Neurochem. Res. 2017, 42, 667–677. [Google Scholar] [CrossRef]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Ye, G.-L.; Du, D.-L.; Jin, L.-J.; Wang, L.-L. Sensitization of TRAIL-resistant cervical cancer cells through combination of TRAIL and fucoxanthin treatments. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5594–5601. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Carotenoids reverse multidrug resistance in cancer cells by interfering with ABC-transporters. Phytomedicine 2012, 19, 977–987. [Google Scholar] [CrossRef]







| Class | Species | Fucoxanthin Yield (mg/g) | References | |
|---|---|---|---|---|
| Macroalgae | Phaeophyceae | Alaria crassifolia | 0.04 a | [18] |
| Cladosiphon okamuranus | - | [19,20] | ||
| Cystoseira hakodatensis | 1.53 a | [18] | ||
| Dictyota coriacea | 6.42 a | [21] | ||
| Eisenia bicyclis | 0.26 b–0.41 a | [18,22] | ||
| Fucus evanescens C. agardh | 0.017 a | [23] | ||
| Fucus vesiculus | 0.26–1.24 | [24] | ||
| Ishige okamurae | - | [25] | ||
| Kjellmaniella crassifolia | 0.197 a | [18] | ||
| Laminaria japonica | 0.03 b–0.19 b | [26,27] | ||
| Myagropsis myagroides | 9.01 a | [21] | ||
| Padina tetrastromatica | 0.18 b | [28] | ||
| Petalonia binghamiae | 0.43 b–0.58 b | [29] | ||
| Saccharina japonica | - | [30] | ||
| Sargassum fusiformis | 0.01 a–0.02 b | [26,27] | ||
| Sargassum binderi | 0.73 a | [31] | ||
| Sargassum duplicatum | 1.01 a | [31] | ||
| Sargassum hemiphyllum | - | [16] | ||
| Sargassum horneri | 1.10 a | [18] | ||
| Sargassum plagyophyllum | 0.71 a | [31] | ||
| Sargassum polycystum | 0.31 a | [32] | ||
| Sargassum siliquastrum | 0.75 a | [33,34] | ||
| Sargassum siliquosum | 1.41 a | [32] | ||
| Scytosiphon lomentaria | 0.24 b–0.56 b | [29] | ||
| Sphaerotrichia divaricata | 0.11 a–1.48 a | [35] | ||
| Turbinaria decurrens | 0.65 a | [36] | ||
| Turbinaria turbinata | 0.59 a | [31] | ||
| Undaria pinnatifida | 0.11 b–1.09 a | [26,27] | ||
| Microalgae | Bacillariophyceae | Cyclotella sp. | 0.7 a–2.3 a | [37] |
| Nitzschia sp. | 4.92 a–5.5 a | [22,37] | ||
| Paralia longispina | 1.4 a | [37] | ||
| Phaeodactylum tricornutum | 8.55 a–24.2 a | [22,37] | ||
| Coccolithophyceae | Prymnesium parvum | 7.91 a | [38] | |
| Chromulinaceae | Chromulina ochromonoides | 1.32 a | [39] | |
| Chrysophyceae | Ochromonas sp. | 0.41 a | [39] | |
| Ochromonas danica | 3.16 a | [38] | ||
| Coscinodiscophyceae | Chaetoceros calcitrans | 5.25 a | [40] | |
| Ochromonas gracilis | 2.24 a | [22] | ||
| Odontella aurita | 21.67 a | [41] | ||
| Mediophyceae | Conticribra weissflogii ND8 | 6 a | [42] | |
| Prymnesiophyceae | Isochrysis sp. | 17 a | [43] | |
| Isochrysis affinis galbana | 18.23 a | [22] | ||
| Isochrysis galbana | 6.04 a | [22] | ||
| Raphidophyceae | Olisthodiscus luteus | 0.08 a | [39] | |
| Synurophyceae | Synura petersenii | 0.02 a | [39] | |
| Mallomonas sp. SBV13 | 26.6 a | [37] | ||
| Poterioochromonas malhamensis | 0.6 a | [39] | ||
| Zygnematophyceae | Cylindrotheca closterium | 5.23 a | [44] |
| Cell Type and Origin | Cell Lines | Concentration (µM) | Effects | References | ||
|---|---|---|---|---|---|---|
| Cancer cells | Lung | Human | NSCLC-N6 A549 | 7.6–60.7 | Apoptosis | [66] |
| A549 H1299 | 12.5–25–50 | Cell cycle arrest (G0/G1 mainly + S) | [67] | |||
| Liver | Human | HepG2 | 1–10 | Apoptosis | [68] | |
| SK-Hep-1 | 1–20 | Cell cycle arrest (G0/G1) Apoptosis | [64] | |||
| HepG2 | 3.8–5.5 | Cell cycle arrest (G1) | [59] | |||
| HepG2 | 25 | Cell cycle arrest (G0/G1) | [62] | |||
| HepG2 | ~20 µg/mL * | Apoptosis | [69] | |||
| Gastric | Human | SGC-7901 | 12.5–25–50 | Apoptosis/Autophagy | [70] | |
| MGC-803 | 50–75 | Cell cycle arrest (G2/M) Apoptosis | [56] | |||
| Colorectal | Human | DLD-1 cells | 5 | Inhibition of epithelial–mesenchymal transition (EMT) | [71] | |
| Caco-2 | 7.6 | Apoptosis | [72] | |||
| HCT116 HT29 | 10–50–100 | DNA damage | [73] | |||
| WiDr | 25–50 | Cell cycle arrest (G0/G1) Apoptosis | [57] | |||
| Caco-2 | 25 | Apoptosis DNA damage | [74] | |||
| Bladder | Human | T24 | 5–10 | Cell cycle arrest (G0/G1) Apoptosis | [54] | |
| EJ-1 | 20 | Apoptosis | [75] | |||
| Prostate | Human | DU145 LNCap | 3.8–5.5 | Cell cycle arrest (G1) | [58,59] | |
| PC-3 DU145 LNCap | 20 | Apoptosis | [76] | |||
| PC-3 | 20 | Apoptosis | [77] | |||
| Breast | Human | MCF 7 MD-MB-231 | 10 | Apoptosis | [78] | |
| MCF 7 | 20 | Protect against DNA damage | [79] | |||
| MCF 7 MD-MB-231 | 20–30–40 | Apoptosis | [80] | |||
| MCF 7 | 25 | Apoptosis/DNA damage | [74] | |||
| Cervix | Human | HeLa | 0.5 | Apoptosis | [81] | |
| Rat | C6 | 6 | Protect against DNA damage | [82] | ||
| Human | HeLa | 10–20–40 | Cell cycle arrest (G0/G1) | [65] | ||
| SiHa | 20 | Apoptosis | [83] | |||
| Neural | Human | GOTO | 7.6–15.2 | G0/G1 arrest | [61] | |
| U251 | 20 | Apoptosis | [84] | |||
| U251/U87 | 25–50 | Apoptosis/Inhibition of migration and invasion | [85] | |||
| Lymphoma | Human | Raji Daudi Ramos BJAB L428 KM-H2 HDLM-2 L540 | 2.5–5 | Cell cycle arrest (G1; at lower concentration) Apoptosis (at higher concentration) | [19] | |
| HHV-8 infected BCBL-1 and TY-1 | 5–10 | Cell cycle arrest (G1) | [53] | |||
| Leukemia | Human | HL-60 | 12.5–25 | Apoptosis/DNA Damage | [74] | |
| HL-60 | 10 | Apoptosis | [86] | |||
| K562 TK6 | 10 | Antiproliferative | [51] | |||
| MT-2/MT-4 HUT-102 ED-40515(-) | 10 | Cell cycle arrest (G1) Apoptosis | [52] | |||
| HL-60 | 15 | Apoptosis | [25] | |||
| Melanoma | Mouse | B16-F10 | 30 | Inhibition of invasion and migration Growth inhibition | [30] | |
| B16-F10 | 50–100–200 | Cell cycle arrest (G0/G1) Apoptosis | [55] | |||
| Sarcoma | Human | Saos 2 | 20 | Apoptosis | [20] | |
| Non-cancer cells | Umbilical vein endothelial cells | Human | HUVEC | 1–5 | Anti-angiogenic | [14] |
| Keratinocytes | HaCaT | 5 ** | Anti-inflammatory | [15] | ||
| Umbilical vein endothelial cells | HUVEC | 2.5–5–10–25–50–100 | Anti-angiogenic | [87] | ||
| Lymphatic endothelial cells | HLEC | 25–50–100 | Anti-angiogenic | [88] | ||
| Context | Dose | Administration | Effects | References | ||
|---|---|---|---|---|---|---|
| Fucoxanthin | Lung | Engrafted with A549 cells | 5–15–50 mg/kg | Oral | Necrosis | [67] |
| Liver | Carcinogenesis model | 0.001% in drinking water | Oral | Inhibition of carcinogenesis | [89] | |
| Duodenal | Carcinogenesis models | 0.005% in drinking water | Oral | Inhibition of carcinogenesis | [90] | |
| Carcinogenesis models | 0.005% in drinking water | Oral | Inhibition of carcinogenesis | [91] | ||
| Colorectal | Carcinogenesis models | 30 mg/kg | Injection (stomach) | Anoikis | [92] | |
| Carcinogenesis models | 0.01% in drinking water | Oral | Inhibition of carcinogenesis | [93] | ||
| Breast | Engrafted with MDA-MB-231 cells | 100 and 500 µmol/L; 100 µL/mouse | Injection | Anti-angiogenic | [88] | |
| Cervix | Engrafted with HeLa cells | 10 and 20 mg/kg | Oral | Growth inhibition | [81] | |
| Lymphoma | Engrafted with B16-F10 | 150 mg/kg | Oral | Growth inhibition | [53] | |
| Melanoma | Engrafted with B16-F10 | 0.1 mg/mouse | Intra-peritoneal injection | Anti-metastasis | [30] | |
| Carcinogenesis models | 200 nM/painting | Topical application (skin painting) | Inhibition of carcinogenesis | [91] | ||
| Sarcoma | Engrafted with S180 cells | 50 and 100 mg/kg | Oral | Apoptosis | [94] | |
| Engrafted with LM8 cells | 200 mg/kg | Oral | Growth inhibition | [20] | ||
| Glioblastoma | Engrafted with U87 cells | 200 mg/kg | Oral | Growth inhibition | [85] | |
| Fucoxanthinol | Lymphoma | Engrafted with HUT-102 cells | 200 mg/kg | Oral | Growth inhibition | [52] |
| Sarcoma | Engrafted with LM8 cells | 200 mg/kg | Oral | Growth inhibition | [20] | |
| Cell Type and Origin | Cell Lines | Concentration (µM) | Effects | References | ||
|---|---|---|---|---|---|---|
| Cancer cells | Colorectal cancer | Human | DLD-1 | 1–5 | Anoikis | [92] |
| DLD-1 | 2.5 | Anoikis Inhibition of EMT | [99] | |||
| HCT116 | 5 | Apoptosis | [98] | |||
| Caco-2 | 12.5–25 | Apoptosis DNA damage | [74] | |||
| CRC HR29 HCT 116 | 50 | Inhibition of EMT | [99] | |||
| Breast cancer | Human | MCF-7 | 12.5–25 | Apoptosis DNA damage | [74] | |
| MCF 7 MDA-MB-231 | 20–30–40 | Apoptosis | [80] | |||
| Lymphoma | Human | Raji Daudi Ramos, BJAB L428 KM-H2 HDLM-2 L540 | 1.25–2.5 | Cell cycle arrest (G1; at lower concentration) Apoptosis (at higher concentration) | [19] | |
| HHV-8 infected BCBL-1 TY-1 | 2.5–5 | Cell cycle arrest (G1) | [53] | |||
| MT-2 MT-4 HUT-102 ED-40515(-) | 5 | Cell cycle arrest (G1) Apoptosis | [52] | |||
| Leukemia | Human | HL-60 | 6.25–12.5 | Apoptosis Antiproliferative DNA Damage | [74] | |
| Sarcoma | Human | Saos 2 | 0.63–1.25 | Inhibition of migration | [20] | |
| 0.05–0.1 | Inhibition of invasion | [20] | ||||
| Non-cancer cells | - | Rat | Aortic ring | 2.5–5–10–25 | Anti-angiogenic | [87] |
| NCT Number | Title | Conditions | Interventions | Phases | Study Designs | Start Date | Locations |
|---|---|---|---|---|---|---|---|
| NCT02875392 | Fucoidan Improves the Metabolic Profiles of Patients With Non-alcoholic Fatty Liver Disease (NAFLD) | Non-alcoholic Fatty Liver Disease | Other: 275 mg Oligo Fucoidan +275 mg HS Fucoxanthin|Other: placebo pills | NA (unknown status) | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Double (Participant, Care Provider) Primary Purpose: Treatment | November 2016 | WanFangH, Taipei, Taiwan |
| NCT03625284 | Oral Dietary Fucoxanthin-Rich Supplement for Liver Health | Non-Alcoholic Fatty Liver | Dietary Supplement: Placebo Dietary Supplement: FucoVital | NA (unknown status) | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) Primary Purpose: Prevention | 10 September 2018 | Assaf-Harofeh Medical Center, Israël |
| NCT03613740 | Effect of Fucoxanthin on the Components of the Metabolic Syndrome, Insulin Sensitivity and Insulin Secretion | Metabolic Syndrome | Drug: Fucoxanthin|Drug: Placebo | Phase 2 (still recruiting) | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Double (Participant, Investigator) Primary Purpose: Treatment | 30 September 2019 | Instituto de Terapéutica Experimental y Clínica. Centro Universitario de Ciencias de la Salud. Guadalajara, Mexico |
| NCT04288544 | Health Promoting Effects of the Microalgae Phaeodactylum Tricornutum | Human Nutrition, Omega-3 Fatty Acids, Microalgae Micronutrients | Dietary Supplement: Microalgae Dietary Supplement: Omega-3-fatty acid capsule Dietary Supplement: sea fish | NA (Enrolling by invitation) | Allocation: Non-Randomized Intervention Model: Crossover Assignment Masking: None (Open Label) Primary Purpose: Supportive Care | 25 February 2020 | lena Stiefvatter, Stuttgart, Germany University of Hohenheim, Stuttgart, Germany |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méresse, S.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9273. https://doi.org/10.3390/ijms21239273
Méresse S, Fodil M, Fleury F, Chénais B. Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy. International Journal of Molecular Sciences. 2020; 21(23):9273. https://doi.org/10.3390/ijms21239273
Chicago/Turabian StyleMéresse, Sarah, Mostefa Fodil, Fabrice Fleury, and Benoît Chénais. 2020. "Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy" International Journal of Molecular Sciences 21, no. 23: 9273. https://doi.org/10.3390/ijms21239273
APA StyleMéresse, S., Fodil, M., Fleury, F., & Chénais, B. (2020). Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy. International Journal of Molecular Sciences, 21(23), 9273. https://doi.org/10.3390/ijms21239273