Mechanisms and Treatment of Light-Induced Retinal Degeneration-Associated Inflammation: Insights from Biochemical Profiling of the Aqueous Humor
Abstract
:1. Introduction
2. Results
2.1. Clinical, Electrophysiological and Morphological Characteristics of the Rabbit Model of LIRD
2.2. Biochemical Manifestations of LIRD-Associated Inflammation in the Aqueous Humor
2.3. Lipidomic Signs of LIRD-Associated Inflammation in the Aqueous Humor
2.4. Functional and Morphological State of the Retina in the Course of Combined Antioxidant/Anti-Inflammatory Treatment of LIRD-Associated Inflammation
2.5. Biochemical and Lipidomic Alterations in the Aqueous Humor in the Course of Combined Antioxidant/Anti-Inflammatory Treatment of LIRD-Associated Inflammation
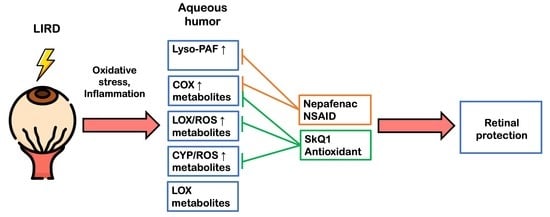
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Ethics Statement
4.3. Experimental Model
4.4. Clinical and Electrophysiological Examination
4.5. Histology
4.6. Aqueous Humor Collection
4.7. Total Protein Concentration Measurement
4.8. Lipid Peroxidation Analysis
4.9. Total Antioxidant Activity Analysis
4.10. Antioxidant Enzyme Activity Analysis
4.11. TNF-α Concentration Measurement
4.12. Lipid Extraction
4.13. UPLC-MS/MS Analysis
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| AH | Aqueous humor |
| AMD | Age-related macular degeneration |
| BCA | Bicinchoninic acid |
| BV | Blood vessel |
| COX | Cyclooxygenases |
| CYP | Cytochrome P450 monooxygenases |
| DHA | Docosahexaenoic acids |
| 9,10-DiHOME | 9,10-dihydroxyoctadecamonoenoic acid |
| 12,13-DiHOME | 12,13-dihydroxyoctadecamonoenoic acid |
| EPA | Eicosapentaenoic acid |
| ERG | Electroretinography |
| 9,10-EpOME | 9,10-epoxyoctadecamonoenoic acid |
| 12,13-EpOME | 12,13-epoxyoctadecamonoenoic acid |
| GCL | Ganglion cell layer |
| 9-HODE | 9-hydroxyoctadecadienoic acid |
| 13-HODE | 13-hydroxyoctadecadienoic acid |
| INL | Inner nuclear retinal layer |
| IL-1 | Interleukin-1 |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1 beta |
| 9-KODE | 9-oxo-octadecadienoic acid |
| 13-KODE | 13-oxo-octadecadienoic acid |
| LTB4 | Leukotriene B4 |
| LIRD | Light-induced retinal degeneration |
| LA | Linoleic acid |
| LOX | Lipoxygenase |
| lyso-PAF | Lyso-Platelet-Activating Factor (1-O-hexadecyl-sn-glyceryl-3-phosphorylcholine) |
| MR | Medullary ray |
| NSAID | Nonsteroidal anti-inflammatory drug |
| ONL | Outer nuclear retinal layer; |
| OEA | Oleoylethanolamine ((Z)-N-(2-Hydroxyethyl)octadec-9-enamide) |
| Ph | Photoreceptor layer |
| PBS | Phosphate buffer saline |
| PAF | Platelet-activating factor |
| PUFA | Polyunsaturated fatty acid |
| PGD2 | Prostaglandin D2 |
| PGE2 | Prostaglandin E2 |
| PGF2α | Prostaglandin F2a |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| Sc | Sclera |
| SkQ1 | 10-(60-plastoquinonyl)-decyltriphenylphosphonium |
| TNF-α | Tumor necrosis factor alpha |
| UPLC-MS/MS | Ultra performance liquid chromatography-tandem mass spectrometry |
References
- Whitcup, S.M.; Nussenblatt, R.B.; Lightman, S.L.; Hollander, D.A. Inflammation in retinal disease. Int. J. Inflamm. 2013, 2013, 724648. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Humphries, P. The blood-retina barrier: Tight junctions and barrier modulation. Adv. Exp. Med. Biol. 2012, 763, 70–84. [Google Scholar]
- Baksheeva, V.E.; Tiulina, V.V.; Tikhomirova, N.K.; Gancharova, O.S.; Komarov, S.V.; Philippov, P.P.; Zamyatnin, A.A., Jr.; Senin, I.I.; Zernii, E.Y. Suppression of Light-Induced Oxidative Stress in the Retina by Mitochondria-Targeted Antioxidant. Antioxidants 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dammann, O. Inflammation and retinopathy of prematurity. Acta Paediatr. 2010, 99, 975–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Ebrahimi, K.B.; Handa, J.T. Lipids, lipoproteins, and age-related macular degeneration. J. Lipids 2011, 2011, 802059. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W. Etiology and treatment of macular edema. Am. J. Ophthalmol. 2009, 147, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Morgan, J.I.; Merigan, W.H.; Sliney, D.H.; Sparrow, J.R.; Williams, D.R. The susceptibility of the retina to photochemical damage from visible light. Prog. Retin. Eye Res. 2012, 31, 28–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zernii, E.Y.; Baksheeva, V.E.; Iomdina, E.N.; Averina, O.A.; Permyakov, S.E.; Philippov, P.P.; Zamyatnin, A.A.; Senin, I.I. Rabbit Models of Ocular Diseases: New Relevance for Classical Approaches. CNS Neurol. Disord.-Drug Targ. 2016, 15, 267–291. [Google Scholar] [CrossRef]
- Byrnes, G.A.; Chang, B.; Loose, I.; Miller, S.A.; Benson, W.E. Prospective incidence of photic maculopathy after cataract surgery. Am. J. Ophthalmol. 1995, 119, 231–232. [Google Scholar] [CrossRef]
- Baksheeva, V.E.; Gancharova, O.S.; Tiulina, V.V.; Iomdina, E.N.; Zamyatnin, A.A., Jr.; Philippov, P.P.; Zernii, E.Y.; Senin, I.I. Iatrogenic Damage of Eye Tissues: Current Problems and Possible Solutions. Biochem. Biokhimiia 2018, 83, 1563–1574. [Google Scholar] [CrossRef]
- Wolffe, M. How safe is the light during ophthalmic diagnosis and surgery. Eye 2016, 30, 186–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, A.; Grimm, C.; Samardzija, M.; Reme, C.E. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 2005, 24, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Zernii, E.Y.; Nazipova, A.A.; Gancharova, O.S.; Kazakov, A.S.; Serebryakova, M.V.; Zinchenko, D.V.; Tikhomirova, N.K.; Senin, I.I.; Philippov, P.P.; Permyakov, E.A.; et al. Light-induced disulfide dimerization of recoverin under ex vivo and in vivo conditions. Free Radic. Biol. Med. 2015, 83, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Zernii, E.Y.; Nazipova, A.A.; Nemashkalova, E.L.; Kazakov, A.S.; Gancharova, O.S.; Serebryakova, M.V.; Tikhomirova, N.K.; Baksheeva, V.E.; Vladimirov, V.I.; Zinchenko, D.V.; et al. Light-Induced Thiol Oxidation of Recoverin Affects Rhodopsin Desensitization. Front. Mol. Neurosci. 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef] [Green Version]
- Geiger, P.; Barben, M.; Grimm, C.; Samardzija, M. Blue light-induced retinal lesions, intraretinal vascular leakage and edema formation in the all-cone mouse retina. Cell. Death Dis. 2015, 6, e1985. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Shang, P.; Yazdankhah, M.; Bhutto, I.; Hose, S.; Montezuma, S.R.; Luo, T.; Chattopadhyay, S.; Qian, J.; Lutty, G.A.; et al. Activating the AKT2-nuclear factor-kappaB-lipocalin-2 axis elicits an inflammatory response in age-related macular degeneration. J. Pathol. 2017, 241, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Zarbin, M.A.; Casaroli-Marano, R.P.; Rosenfeld, P.J. Age-related macular degeneration: Clinical findings, histopathology and imaging techniques. Dev. Ophthalmol. 2014, 53, 1–32. [Google Scholar] [CrossRef]
- Wooff, Y.; Man, S.M.; Aggio-Bruce, R.; Natoli, R.; Fernando, N. IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases. Front. Immunol. 2019, 10, 1618. [Google Scholar] [CrossRef]
- Mirshahi, A.; Hoehn, R.; Lorenz, K.; Kramann, C.; Baatz, H. Anti-tumor necrosis factor alpha for retinal diseases: Current knowledge and future concepts. J. Ophthalmic Vis. Res. 2012, 7, 39–44. [Google Scholar]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Q.; Wang, Y.; Wang, C.; Ji, B.; Cong, R.; Zhao, L.; Chen, P.; Zang, X.; Lu, F.; Han, F.; et al. Dietary supplementation with omega-3 polyunsaturated fatty acid-rich oils protects against visible-light-induced retinal damage in vivo. Food Funct. 2018, 9, 2469–2479. [Google Scholar] [CrossRef]
- Kim, G.H.; Paik, S.S.; Park, Y.S.; Kim, H.G.; Kim, I.B. Amelioration of Mouse Retinal Degeneration After Blue LED Exposure by Glycyrrhizic Acid-Mediated Inhibition of Inflammation. Front. Cell. Neurosci. 2019, 13, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toris, C.B.; Gulati, V. The biology, pathology and therapeutic use of prostaglandins in the eye. Clin. Lipidol. 2011, 6, 577–591. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenberger, S.D.; Kim, S.J. Nonsteroidal anti-inflammatory drugs for retinal disease. Int. J. Inflamm. 2013, 2013, 281981. [Google Scholar] [CrossRef]
- Bazan, N.G.; Reddy, T.S.; Bazan, H.E.; Birkle, D.L. Metabolism of arachidonic and docosahexaenoic acids in the retina. Prog. Lipid Res. 1986, 25, 595–606. [Google Scholar] [CrossRef]
- Tanito, M.; Yoshida, Y.; Kaidzu, S.; Ohira, A.; Niki, E. Detection of lipid peroxidation in light-exposed mouse retina assessed by oxidative stress markers, total hydroxyoctadecadienoic acid and 8-iso-prostaglandin F-2 alpha. Neurosci. Lett. 2006, 398, 63–68. [Google Scholar] [CrossRef]
- Morrow, J.D.; Awad, J.A.; Boss, H.J.; Blair, I.A.; Roberts, L.J., 2nd. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. USA 1992, 89, 10721–10725. [Google Scholar] [CrossRef] [Green Version]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubenstein, D.A.; Yin, W.; Frame, M.D. Biofluid Mechanics: An Introduction to Fluid Mechanics, Macrocirculation, and Microcirculation, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Boston, MA, USA, 2015; p. xii. 531p. [Google Scholar]
- Mo, J.S.; Wang, W.; Kaplan, H.J. Impact of inflammation on ocular immune privilege. Chem. Immunol. Allergy 2007, 92, 155–165. [Google Scholar] [CrossRef]
- Richer, S.P.; Rose, R.C. Water soluble antioxidants in mammalian aqueous humor: Interaction with UV B and hydrogen peroxide. Vis. Res. 1998, 38, 2881–2888. [Google Scholar] [CrossRef] [Green Version]
- Nucci, C.; Di Pierro, D.; Varesi, C.; Ciuffoletti, E.; Russo, R.; Gentile, R.; Cedrone, C.; Pinazo Duran, M.D.; Coletta, M.; Mancino, R. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol. Vis. 2013, 19, 1841–1846. [Google Scholar] [PubMed]
- McGahan, M.C.; Fleisher, L.N. Antioxidant activity of aqueous and vitreous humor from the inflamed rabbit eye. Curr. Eye Res. 1986, 5, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Satici, A.; Guzey, M.; Gurler, B.; Vural, H.; Gurkan, T. Malondialdehyde and antioxidant enzyme levels in the aqueous humor of rabbits in endotoxin-induced uveitis. Eur. J. Ophthalmol. 2003, 13, 779–783. [Google Scholar] [CrossRef]
- Beyazyildiz, E.; Cankaya, A.B.; Beyazyildiz, O.; Ergan, E.; Celik, H.T.; Yilmazbas, P.; Ozturk, F. Disturbed oxidant/antioxidant balance in aqueous humour of patients with exfoliation syndrome. Jpn. J. Ophthalmol. 2014, 58, 353–358. [Google Scholar] [CrossRef]
- Terao, N.; Koizumi, H.; Kojima, K.; Yamagishi, T.; Yamamoto, Y.; Yoshii, K.; Kitazawa, K.; Hiraga, A.; Toda, M.; Kinoshita, S.; et al. Distinct Aqueous Humour Cytokine Profiles of Patients with Pachychoroid Neovasculopathy and Neovascular Age-related Macular Degeneration. Sci. Rep. 2018, 8, 10520. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.F.; Yu, H.H.; Yu, Y.; Geng, Y.; Li, D.L.; Yang, C.; Lv, Q.J.; Lu, L.; Liu, T.; Li, G.D.; et al. Levels of Inflammatory Cytokines IL-1 beta, IL-6, IL-8, IL-17A, and TNF-alpha in Aqueous Humour of Patients with Diabetic Retinopathy. J. Diabetes Res. 2018, 2018, 8546423. [Google Scholar] [CrossRef] [Green Version]
- Hillier, R.J.; Ojaimi, E.; Wong, D.T.; Mak, M.Y.K.; Berger, A.R.; Kohly, R.P.; Kertes, P.J.; Forooghian, F.; Boyd, S.R.; Eng, K.; et al. Aqueous Humor Cytokine Levels as Biomarkers of Disease Severity in Diabetic Macular Edema. Retin. J. Ret. Vit. Dis. 2017, 37, 761–769. [Google Scholar] [CrossRef]
- Takai, Y.; Tanito, M.; Ohira, A. Multiplex Cytokine Analysis of Aqueous Humor in Eyes with Primary Open-Angle Glaucoma, Exfoliation Glaucoma, and Cataract. Investig. Ophthalmol. Vis. Sci. 2012, 53, 241–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novikova, Y.P.; Gancharova, O.S.; Eichler, O.V.; Philippov, P.P.; Grigoryan, E.N. Preventive and therapeutic effects of SkQ1-containing Visomitin eye drops against light-induced retinal degeneration. Biochemistry 2014, 79, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Ke, T.L.; Graff, G.; Spellman, J.M.; Yanni, J.M. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation 2000, 24, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. CMLS 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sennlaub, F.; Auvynet, C.; Calippe, B.; Lavalette, S.; Poupel, L.; Hu, S.J.; Dominguez, E.; Camelo, S.; Levy, O.; Guyon, E.; et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol. Med. 2013, 5, 1775–1793. [Google Scholar] [CrossRef]
- Yorston, D. What’s new in age-related macular degeneration? Community Eye Health 2006, 19, 4–5. [Google Scholar]
- El Baba, F.; Jarrett, W.H., 2nd; Harbin, T.S., Jr.; Fine, S.L.; Michels, R.G.; Schachat, A.P.; Green, W.R. Massive hemorrhage complicating age-related macular degeneration. Clinicopathologic correlation and role of anticoagulants. Ophthalmology 1986, 93, 1581–1592. [Google Scholar] [CrossRef]
- Spindler, J.; Zandi, S.; Pfister, I.B.; Gerhardt, C.; Garweg, J.G. Cytokine profiles in the aqueous humor and serum of patients with dry and treated wet age-related macular degeneration. PLoS ONE 2018, 13, e0203337. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Dick, A.D.; Brezin, A.P.; Nguyen, Q.D.; Thorne, J.E.; Kestelyn, P.; Barisani-Asenbauer, T.; Franco, P.; Heiligenhaus, A.; Scales, D.; et al. Adalimumab in Patients with Active Noninfectious Uveitis. New Engl. J. Med. 2016, 375, 932–943. [Google Scholar] [CrossRef] [Green Version]
- Sacca, S.C.; Roszkowska, A.M.; Izzotti, A. Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases. Mutat. Res. 2013, 752, 153–171. [Google Scholar] [CrossRef]
- Dolz-Marco, R.; Gallego-Pinazo, R.; Pinazo-Duran, M.D.; Pons-Vazquez, S.; Domingo-Pedro, J.C.; Diaz-Llopis, M. Intravitreal docosahexaenoic acid in a rabbit model: Preclinical safety assessment. PLoS ONE 2014, 9, e96872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanito, M.; Anderson, R.E. Dual roles of polyunsaturated fatty acids in retinal physiology and pathophysiology associated with retinal degeneration. Clin. Lipidol. 2009, 4, 821–827. [Google Scholar] [CrossRef]
- Thivierge, M.; Rola-Pleszczynski, M. Up-regulation of inducible cyclooxygenase gene expression by platelet-activating factor in activated rat alveolar macrophages. J. Immunol. 1995, 154, 6593–6599. [Google Scholar] [PubMed]
- Elison, J.R.; Weinstein, J.E.; Sheets, K.G.; Regan, C.E.; Lentz, J.J.; Reinoso, M.; Gordon, W.C.; Bazan, N.G. Platelet-Activating Factor (PAF) Receptor Antagonism Modulates Inflammatory Signaling in Experimental Uveitis. Curr. Eye Res. 2018, 43, 821–827. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Angell, E.; Wilson, D.; Broquet, C.; Boney, R.S.; Braquet, P. Intravitreally injected platelet activating factor induces retinitis in experimental animals. Curr. Eye Res. 1999, 18, 342–348. [Google Scholar] [CrossRef]
- Secchi, A.G.; Fregona, I.; D’Ermo, F. Lysophosphatidyl choline in the aqueous humour during ocular inflammation. Br. J. Ophthalmol. 1979, 63, 768–770. [Google Scholar] [CrossRef] [Green Version]
- Eakins, K.E.; Whitelocke, R.A.; Perkins, E.S.; Bennett, A.; Unger, W.G. Release of prostaglandins in ocular inflammation in the rabbit. Nat. New Biol. 1972, 239, 248–249. [Google Scholar] [CrossRef]
- Miller, J.D.; Eakins, K.E.; Atwal, M. The release of PGE2-like activity into aqueous humor after paracentesis and its prevention by aspirin. Investig. Ophthalmol. 1973, 12, 939–942. [Google Scholar]
- Unger, W.G.; Perkins, E.S.; Bass, M.S. The response of the rabbit eye to laser irradiation of the iris. Exp. Eye Res. 1974, 19, 367–377. [Google Scholar] [CrossRef]
- Csukas, S.; Paterson, C.A.; Brown, K.; Bhattacherjee, P. Time course of rabbit ocular inflammatory response and mediator release after intravitreal endotoxin. Investig. Ophthalmol. Vis. Sci. 1990, 31, 382–387. [Google Scholar]
- Chen, E.; Benz, M.S.; Fish, R.H.; Brown, D.M.; Wong, T.P.; Kim, R.Y.; Major, J.C. Use of nepafenac (Nevanac) in combination with intravitreal anti-VEGF agents in the treatment of recalcitrant exudative macular degeneration requiring monthly injections. Clin. Ophthalmol. 2010, 4, 1249–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acar, U.; Acar, D.E.; Tanriverdi, C.; Acar, M.; Ozdemir, O.; Erikci, A.; Ornek, F. Prostaglandin E2 Levels of Aqueous and Vitreous Humor in Ketorolac 0.4% and Nepafenac 0.1% Administered Healthy Rabbits. Ocul. Immunol. Inflamm. 2017, 25, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Umeno, A.; Shichiri, M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J. Clin. Biochem. Nutr. 2013, 52, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxid. Med. Cell. Longev. 2017, 9208489. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.J.; Du, X.Y.; Cui, J.G.; Wang, P.W.; Wang, W.J.; Zhu, W.L.; Zhang, T.; Chen, Y. Celastrol protects mouse retinas from bright light-induced degeneration through inhibition of oxidative stress and inflammation. J. Neuroinflamm. 2016, 13, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, S.; Kurihara, T.; Ebinuma, M.; Kubota, M.; Yuki, K.; Sasaki, M.; Noda, K.; Ozawa, Y.; Oike, Y.; Ishida, S.; et al. Resveratrol Prevents Light-Induced Retinal Degeneration via Suppressing Activator Protein-1 Activation. Am. J. Pathol. 2010, 177, 1725–1731. [Google Scholar] [CrossRef]
- Zernii, E.Y.; Golovastova, M.O.; Baksheeva, V.E.; Kabanova, E.I.; Ishutina, I.E.; Gancharova, O.S.; Gusev, A.E.; Savchenko, M.S.; Loboda, A.P.; Sotnikova, L.F.; et al. Alterations in tear biochemistry associated with postanesthetic chronic dry eye syndrome. Biochemistry 2016, 81, 1549–1557. [Google Scholar] [CrossRef]
- Zernii, E.Y.; Gancharova, O.S.; Baksheeva, V.E.; Golovastova, M.O.; Kabanova, E.I.; Savchenko, M.S.; Tiulina, V.V.; Sotnikova, L.F.; Zamyatnin, A.A.; Philippov, P.P.; et al. Mitochondria-Targeted Antioxidant SkQ1 Prevents Anesthesia-Induced Dry Eye Syndrome. Oxid. Med. Cell. Longev. 2017, 9281519. [Google Scholar] [CrossRef]
- Zernii, E.Y.; Gancharova, O.S.; Tiulina, V.V.; Zamyatnin, A.A., Jr.; Philippov, P.P.; Baksheeva, V.E.; Senin, I.I. Mitochondria-targeted antioxidant SKQ1 protects cornea from oxidative damage induced by ultraviolet irradiation and mechanical injury. BMC Ophthalmol. 2018, 18, 336. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Grabeklis, S.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G.; Reiser, G. Astrocytes synthesize primary and cyclopentenone prostaglandins that are negative regulators of their proliferation. Biochem. Biophys. Res. Commun. 2018, 500, 204–210. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chistyakov, D.V.; Baksheeva, V.E.; Tiulina, V.V.; Goriainov, S.V.; Azbukina, N.V.; Gancharova, O.S.; Arifulin, E.A.; Komarov, S.V.; Chistyakov, V.V.; Tikhomirova, N.K.; et al. Mechanisms and Treatment of Light-Induced Retinal Degeneration-Associated Inflammation: Insights from Biochemical Profiling of the Aqueous Humor. Int. J. Mol. Sci. 2020, 21, 704. https://doi.org/10.3390/ijms21030704
Chistyakov DV, Baksheeva VE, Tiulina VV, Goriainov SV, Azbukina NV, Gancharova OS, Arifulin EA, Komarov SV, Chistyakov VV, Tikhomirova NK, et al. Mechanisms and Treatment of Light-Induced Retinal Degeneration-Associated Inflammation: Insights from Biochemical Profiling of the Aqueous Humor. International Journal of Molecular Sciences. 2020; 21(3):704. https://doi.org/10.3390/ijms21030704
Chicago/Turabian StyleChistyakov, Dmitry V., Viktoriia E. Baksheeva, Veronika V. Tiulina, Sergei V. Goriainov, Nadezhda V. Azbukina, Olga S. Gancharova, Eugene A. Arifulin, Sergey V. Komarov, Viktor V. Chistyakov, Natalia K. Tikhomirova, and et al. 2020. "Mechanisms and Treatment of Light-Induced Retinal Degeneration-Associated Inflammation: Insights from Biochemical Profiling of the Aqueous Humor" International Journal of Molecular Sciences 21, no. 3: 704. https://doi.org/10.3390/ijms21030704
APA StyleChistyakov, D. V., Baksheeva, V. E., Tiulina, V. V., Goriainov, S. V., Azbukina, N. V., Gancharova, O. S., Arifulin, E. A., Komarov, S. V., Chistyakov, V. V., Tikhomirova, N. K., Zamyatnin, A. A., Jr., Philippov, P. P., Senin, I. I., Sergeeva, M. G., & Zernii, E. Y. (2020). Mechanisms and Treatment of Light-Induced Retinal Degeneration-Associated Inflammation: Insights from Biochemical Profiling of the Aqueous Humor. International Journal of Molecular Sciences, 21(3), 704. https://doi.org/10.3390/ijms21030704