Effect of Pharmacological Inhibition of the Catalytic Activity of Phosphatases of Regenerating Liver in Early T Cell Receptor Signaling Dynamics and IL-2 Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Expression and Subcellular Distribution of PRL-3 in T Cells
2.2. Delivery of GFP-PRL-3 to the IS
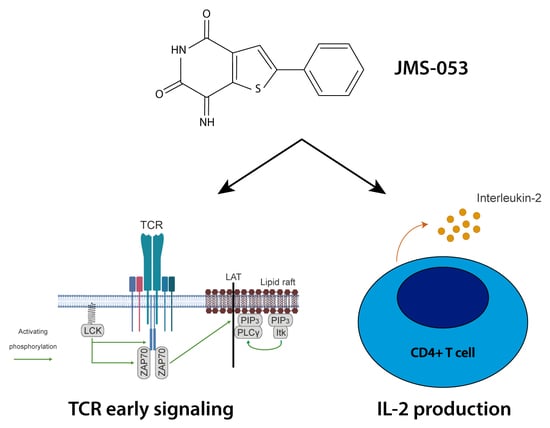
2.3. Catalytic Activity of PRLs Regulates the Dynamics of Antigen-Induced Early Signaling and IL-2 Secretion
3. Materials and Methods
3.1. Cells
3.2. Antibodies and Reagents
3.3. Plasmids and Transfection
3.4. Stimulation of JK Cells for Western Blot and ELISA Assays
3.5. Western Blot
3.6. Enzyme-Linked Immunoabsorbent Assay (ELISA)
3.7. Immunofluorescence and Confocal Microscopy
3.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dustin, M.L.; Choudhuri, K. Signaling and Polarized Communication Across the T Cell Immunological Synapse. Annu. Rev. Cell Dev. Biol. 2016, 32, 303–325. [Google Scholar] [CrossRef] [PubMed]
- Stanford, S.M.; Rapini, N.; Bottini, N. Regulation of TCR signaling by tyrosine phosphatases: From immune homeostasis to autoimmunity. Immunology 2012, 137, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sanchez, P.; Aguilar-Sopena, O.; Alegre-Gomez, S.; Ramirez-Munoz, R.; Roda-Navarro, P. Regulation of CD4(+) T Cell Signaling and Immunological Synapse by Protein Tyrosine Phosphatases: Molecular Mechanisms in Autoimmunity. Front. Immunol. 2019, 10, 1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessette, D.C.; Qiu, D.; Pallen, C.J. PRL PTPs: Mediators and markers of cancer progression. Cancer Metastasis Rev. 2008, 27, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.; Kostantin, E.; Hatzihristidis, T.; Zolotarov, Y.; Uetani, N.; Tremblay, M.L. Physiological and oncogenic roles of the PRL phosphatases. FEBS J. 2018, 285, 3886–3908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiordalisi, J.J.; Keller, P.J.; Cox, A.D. PRL tyrosine phosphatases regulate rho family GTPases to promote invasion and motility. Cancer Res. 2006, 66, 3153–3161. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, M.; Lazo, J.S. Phosphatase of regenerating liver-1 promotes cell migration and invasion and regulates filamentous actin dynamics. J. Pharmacol. Exp. Ther. 2010, 334, 627–633. [Google Scholar] [CrossRef] [Green Version]
- McParland, V.; Varsano, G.; Li, X.; Thornton, J.; Baby, J.; Aravind, A.; Meyer, C.; Pavic, K.; Rios, P.; Kohn, M. The metastasis-promoting phosphatase PRL-3 shows activity toward phosphoinositides. Biochemistry 2011, 50, 7579–7590. [Google Scholar] [CrossRef]
- Wang, J.; Kirby, C.E.; Herbst, R. The tyrosine phosphatase PRL-1 localizes to the endoplasmic reticulum and the mitotic spindle and is required for normal mitosis. J. Biol. Chem. 2002, 277, 46659–46668. [Google Scholar] [CrossRef] [Green Version]
- Comrie, W.A.; Burkhardt, J.K. Action and Traction: Cytoskeletal Control of Receptor Triggering at the Immunological Synapse. Front. Immunol. 2016, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Huse, M. Mechanical forces in the immune system. Nat. Rev. Immunol. 2017, 17, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sanchez, P.; Ramirez-Munoz, R.; Martin-Cofreces, N.B.; Aguilar-Sopena, O.; Alegre-Gomez, S.; Hernandez-Perez, S.; Reyes, R.; Zeng, Q.; Cabanas, C.; Sanchez-Madrid, F.; et al. Phosphatase of Regenerating Liver-1 (PRL-1) Regulates Actin Dynamics During Immunological Synapse Assembly and T Cell Effector Function. Front. Immunol. 2018, 9, 2655. [Google Scholar] [CrossRef] [PubMed]
- McQueeney, K.E.; Salamoun, J.M.; Ahn, J.G.; Pekic, P.; Blanco, I.K.; Struckman, H.L.; Sharlow, E.R.; Wipf, P.; Lazo, J.S. A chemical genetics approach identifies PTP4A3 as a regulator of colon cancer cell adhesion. FASEB J. 2018, 32, 5661–5673. [Google Scholar] [CrossRef] [PubMed]
- Lazo, J.S.; Blanco, I.K.; Tasker, N.R.; Rastelli, E.J.; Burnett, J.C.; Garrott, S.R.; Hart, D.J.; McCloud, R.L.; Hsu, K.L.; Wipf, P.; et al. Next-Generation Cell-Active Inhibitors of the Undrugged Oncogenic PTP4A3 Phosphatase. J. Pharmacol. Exp. Ther. 2019, 371, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Haney, M.G.; Rivas, D.R.; Blackburn, J.S. Protein tyrosine phosphatase 4A3 (PTP4A3/PRL-3) drives migration and progression of T-cell acute lymphoblastic leukemia in vitro and in vivo. Oncogenesis 2020, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Hjort, M.A.; Hov, H.; Abdollahi, P.; Vandsemb, E.N.; Fagerli, U.M.; Lund, B.; Slordahl, T.S.; Borset, M.; Ro, T.B. Phosphatase of regenerating liver-3 (PRL-3) is overexpressed in classical Hodgkin lymphoma and promotes survival and migration. Exp. Hematol. Oncol. 2018, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Das, V.; Nal, B.; Dujeancourt, A.; Thoulouze, M.I.; Galli, T.; Roux, P.; Dautry-Varsat, A.; Alcover, A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity 2004, 20, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Nickel, W. Pathways of unconventional protein secretion. Curr. Opin. Biotechnol. 2010, 21, 621–626. [Google Scholar] [CrossRef]
- Liu, H.; Rhodes, M.; Wiest, D.L.; Vignali, D.A. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity 2000, 13, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Vardy, L.A.; Tan, C.P.; Loo, J.M.; Guo, K.; Li, J.; Lim, S.G.; Zhou, J.; Chng, W.J.; Ng, S.B.; et al. PCBP1 suppresses the translation of metastasis-associated PRL-3 phosphatase. Cancer Cell 2010, 18, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Huse, M.; Lillemeier, B.F.; Kuhns, M.S.; Chen, D.S.; Davis, M.M. T cells use two directionally distinct pathways for cytokine secretion. Nat. Immunol. 2006, 7, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Onnis, A.; Baldari, C.T. Orchestration of Immunological Synapse Assembly by Vesicular Trafficking. Front. Cell. Dev. Biol. 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQueeney, K.E.; Salamoun, J.M.; Burnett, J.C.; Barabutis, N.; Pekic, P.; Lewandowski, S.L.; Llaneza, D.C.; Cornelison, R.; Bai, Y.; Zhang, Z.Y.; et al. Targeting ovarian cancer and endothelium with an allosteric PTP4A3 phosphatase inhibitor. Oncotarget 2018, 9, 8223–8240. [Google Scholar] [CrossRef]
- Daouti, S.; Li, W.H.; Qian, H.; Huang, K.S.; Holmgren, J.; Levin, W.; Reik, L.; McGady, D.L.; Gillespie, P.; Perrotta, A.; et al. A selective phosphatase of regenerating liver phosphatase inhibitor suppresses tumor cell anchorage-independent growth by a novel mechanism involving p130Cas cleavage. Cancer Res. 2008, 68, 1162–1169. [Google Scholar] [CrossRef] [Green Version]
- Stadlbauer, S.; Rios, P.; Ohmori, K.; Suzuki, K.; Kohn, M. Procyanidins Negatively Affect the Activity of the Phosphatases of Regenerating Liver. PLoS ONE 2015, 10, e0134336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Zhou, H.M.; Zhang, L.; Dong, Y.; Zeng, Q.; Shou, W.; Zhang, Z.Y. Role of phosphatase of regenerating liver 1 (PRL1) in spermatogenesis. Sci. Rep. 2016, 6, 34211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wei, Y.; Liu, Y.; Guan, W.; Zhang, X.; Kong, J.; Li, H.; Yang, S.; Wang, H. Metastatic Phosphatase PRL-3 Induces Ovarian Cancer Stem Cell Sub-population through Phosphatase-Independent Deacetylation Modulations. iScience 2020, 23, 100766. [Google Scholar] [CrossRef] [Green Version]
- Kanellopoulou, C.; George, A.B.; Masutani, E.; Cannons, J.L.; Ravell, J.C.; Yamamoto, T.N.; Smelkinson, M.G.; Jiang, P.D.; Matsuda-Lennikov, M.; Reilley, J.; et al. Mg2+ regulation of kinase signaling and immune function. J. Exp. Med. 2019, 216, 1828–1842. [Google Scholar] [CrossRef]
- Salter, R.D.; Howell, D.N.; Cresswell, P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics 1985, 21, 235–246. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Sopeña, O.; Hernández-Pérez, S.; Alegre-Gómez, S.; Castro-Sánchez, P.; Iglesias-Ceacero, A.; Lazo, J.S.; Roda-Navarro, P. Effect of Pharmacological Inhibition of the Catalytic Activity of Phosphatases of Regenerating Liver in Early T Cell Receptor Signaling Dynamics and IL-2 Production. Int. J. Mol. Sci. 2020, 21, 2530. https://doi.org/10.3390/ijms21072530
Aguilar-Sopeña O, Hernández-Pérez S, Alegre-Gómez S, Castro-Sánchez P, Iglesias-Ceacero A, Lazo JS, Roda-Navarro P. Effect of Pharmacological Inhibition of the Catalytic Activity of Phosphatases of Regenerating Liver in Early T Cell Receptor Signaling Dynamics and IL-2 Production. International Journal of Molecular Sciences. 2020; 21(7):2530. https://doi.org/10.3390/ijms21072530
Chicago/Turabian StyleAguilar-Sopeña, Oscar, Sara Hernández-Pérez, Sergio Alegre-Gómez, Patricia Castro-Sánchez, Alba Iglesias-Ceacero, John S. Lazo, and Pedro Roda-Navarro. 2020. "Effect of Pharmacological Inhibition of the Catalytic Activity of Phosphatases of Regenerating Liver in Early T Cell Receptor Signaling Dynamics and IL-2 Production" International Journal of Molecular Sciences 21, no. 7: 2530. https://doi.org/10.3390/ijms21072530
APA StyleAguilar-Sopeña, O., Hernández-Pérez, S., Alegre-Gómez, S., Castro-Sánchez, P., Iglesias-Ceacero, A., Lazo, J. S., & Roda-Navarro, P. (2020). Effect of Pharmacological Inhibition of the Catalytic Activity of Phosphatases of Regenerating Liver in Early T Cell Receptor Signaling Dynamics and IL-2 Production. International Journal of Molecular Sciences, 21(7), 2530. https://doi.org/10.3390/ijms21072530