Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration
Abstract
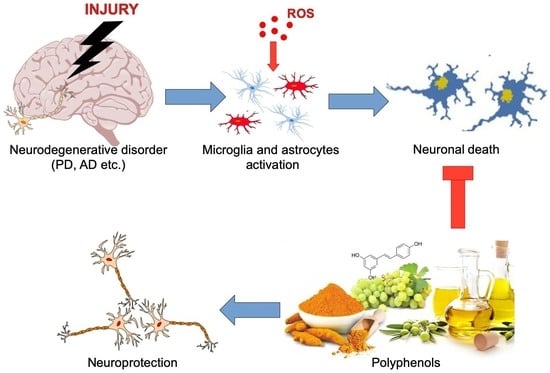
:1. Introduction
2. Methodology
3. Oxidative Stress and Polyphenols
4. Molecular Pathways Involved in Neuroprotection of Polyphenols
5. Effect of Polyphenols on Neurodegenerative Disorders
5.1. Alzheimer’s Disease
5.2. Parkinson’s Disease
5.3. Huntington’s Disease and Vascular Dementia
6. Bioactivity of Polyphenols and Gut Microbiota Interplay
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Fernandez, L.; Comes, G.; Bolea, I.; Valente, T.; Ruiz, J.; Murtra, P.; Ramirez, B.; Angles, N.; Reguant, J.; Morello, J.; et al. LMN diet, rich in polyphenols and polyunsaturated fatty acids, improves mouse cognitive decline associated with aging and Alzheimer’s disease. Behav. Brain Res. 2012, 228, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Kemper, L.; Wang, J.; Zahs, K.; Ashe, K.; Pasinetti, G. Grape seed polyphenolic extract specifically decreases Aβ* 56 in the brains of Tg2576 mice. J. Alzheimers Dis. 2011, 26, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, D.; Rhodes, D.; McVey, D.S.; Fawver, Z.; Perumal, R.; Herald, T.; Noronha, L. High-polyphenol sorghum bran extract inhibits cancer cell growth through ROS induction, cell cycle arrest, and apoptosis. J. Med. Food 2018, 21, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Mello-Filho, A.C.; Meneghini, R. Iron is the intracellular metal involved in the production of DNA damage by oxygen radicals. Mutat. Res. 1991, 251, 109–113. [Google Scholar] [CrossRef]
- Gupta, S.; Hastak, K.; Afaq, F.; Ahmad, N.; Mukhtar, H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappa B and induction of apoptosis. Oncogene 2004, 23, 2507–2522. [Google Scholar] [CrossRef] [Green Version]
- Kinarivala, N.; Patel, R.; Boustany, R.M.; Al-Ahmad, A.; Trippier, P.C. Discovery of aromatic carbamates that confer neuroprotective activity by enhancing autophagy and inducing the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2). J. Med. Chem. 2017, 60, 9739–9756. [Google Scholar] [CrossRef]
- Nakaso, K.; Ito, S.; Nakashima, K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson’s disease model of SH-SY5Y cells. Neurosci. Lett. 2008, 432, 146–150. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rice-Evans, C.; Williams, R.J.; Spencer, J.P. Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J. Neurochem. 2007, 103, 1355–1367. [Google Scholar] [CrossRef]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. 2016, 10, 23–42. [Google Scholar]
- Di Meo, F.; Donato, S.; Di Pardo, A.; Maglione, V.; Filosa, S.; Crispi, S. New therapeutic drugs from bioactive natural molecules: The role of gut microbiota metabolism in neurodegenerative diseases. Curr. Drug Metab. 2018, 19, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Filosa, S.; Fico, A.; Paglialunga, F.; Balestrieri, M.; Crooke, A.; Verde, P.; Abrescia, P.; Bautista, J.M.; Martini, G. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem. J. 2003, 370, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Fico, A.; Paglialunga, F.; Cigliano, L.; Abrescia, P.; Verde, P.; Martini, G.; Iaccarino, I.; Filosa, S. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ. 2004, 11, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Antioxidant characterization. Methodology and mechanism. Biochem. Pharm. 1995, 49, 1341–1348. [Google Scholar] [CrossRef]
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Ageing, neuroinflammation and neurodegeneration. Front. Biosci. 2015, 7, 189–204. [Google Scholar] [CrossRef]
- Matés, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Qin, S.; Hou, D.X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res. 2016, 60, 1731–1755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Gheldof, N.; Engeseth, N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef]
- Del Bano, M.J.; Lorente, J.; Castillo, J.; Benavente-Garcia, O.; del Rio, J.A.; Ortuno, A.; Quirin, K.W.; Gerard, D. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxid. Act. J. Agric. Food Chem. 2003, 51, 4247–4253. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Foley, T.D. Reductive reprogramming: A not-so-radical hypothesis of neurodegeneration linking redox perturbations to neuroinflammation and excitotoxicity. Cell Mol. Neurobiol. 2019, 39, 577–590. [Google Scholar] [CrossRef]
- Cui, K.; Luo, X.; Xu, K.; Ven Murthy, M.R. Role of oxidative stress in neurodegeneration: Recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 771–799. [Google Scholar] [CrossRef]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, S18–S25. [Google Scholar] [CrossRef]
- Mattson, M.P.; Magnus, T. Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 2006, 7, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Lee, K.S.; Lee, S.; Park, J.H.; Choi, H.E.; Go, S.H.; Kwak, H.J.; Park, H.Y. 15d-PGJ2 stimulates HO-1 expression through p38 MAP kinase and Nrf-2 pathway in rat vascular smooth muscle cells. Toxicol. Appl. Pharm. 2007, 223, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Son, T.G.; Park, H.R.; Park, M.; Kim, M.S.; Kim, H.S.; Chung, H.Y.; Mattson, M.P.; Lee, J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J. Biol. Chem. 2008, 283, 14497–14505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhakkiyalakshmi, E.; Dineshkumar, K.; Karthik, S.; Sireesh, D.; Hopper, W.; Paulmurugan, R.; Ramkumar, K.M. Pterostilbene-mediated Nrf2 activation: Mechanistic insights on Keap1: Nrf2 interface. Bioorg. Med. Chem. 2016, 24, 3378–3386. [Google Scholar] [CrossRef]
- Lu, B.; Chow, A. Neurotrophins and hippocampal synaptic transmission and plasticity. J. Neurosci. Res. 1999, 58, 76–87. [Google Scholar] [CrossRef]
- Chao, M.V.; Rajagopal, R.; Lee, F.S. Neurotrophin signalling in health and disease. Clin. Sci. 2006, 110, 167–173. [Google Scholar] [CrossRef]
- Sariola, H.; Saarma, M. Novel functions and signalling pathways for GDNF. J. Cell Sci. 2003, 116, 3855–3862. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Niisato, N.; Marunaka, Y. Quercetin stimulates NGF-induced neurite outgrowth in PC12 cells via activation of Na(+)/K(+)/2Cl(−) cotransporter. Cell Physiol. Biochem. 2011, 28, 147–156. [Google Scholar] [CrossRef]
- Nakajima, K.; Niisato, N.; Marunaka, Y. Genistein enhances the NGF-induced neurite outgrowth. Biomed. Res. 2011, 32, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wang, Y.Y.; Liu, H.; Lu, Y.F.; Wu, Q.; Liu, J.; Shi, J.S. Resveratrol produces neurotrophic effects on cultured dopaminergic neurons through prompting astroglial BDNF and GDNF release. Evid. Based Complement. Altern. Med. 2012, 2012, 937605. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Liu, H.; Li, Z. The protective effects of resveratrol on Schwann cells with toxicity induced by ethanol in vitro. Neurochem. Int. 2013, 63, 146–153. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. TRK receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chan, C.B.; Ye, K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl. Neurodegener. 2016, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Liu, Y.; Cheng, M. Resveratrol protects bupivacaine-induced neuro-apoptosis in dorsal root ganglion neurons via activation on tropomyosin receptor kinase A. Biomed. Pharm. 2018, 103, 1545–1551. [Google Scholar] [CrossRef]
- Spencer, J.P.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Si, T.L.; Liu, Q.; Ren, Y.F.; Li, H.; Xu, X.Y.; Li, E.H.; Pan, S.Y.; Zhang, J.L.; Wang, K.X. Enhanced anti-inflammatory effects of DHA and quercetin in lipopolysaccharide-induced RAW264.7 macrophages by inhibiting NF-κB and MAPK activation. Mol. Med. Rep. 2016, 14, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Zhu, X.; Raina, A.K.; Lee, H.G.; Casadesus, G.; Smith, M.A.; Perry, G. Oxidative stress signalling in Alzheimer’s disease. Brain Res. 2004, 1000, 32–39. [Google Scholar] [CrossRef]
- Di Pardo, A.; Amico, E.; Scalabrì, F.; Pepe, G.; Castaldo, S.; Elifani, F.; Capocci, L.; De Sanctis, C.; Comerci, L.; Pompeo, F.; et al. Impairment of blood-brain barrier is an early event in R6/2 mouse model of Huntington’s disease. Sci. Rep. 2017, 7, 41316. [Google Scholar] [CrossRef]
- Knutson, M.D.; Leeuwenburgh, C. Resveratrol and novel potent activators of SIRT1: Effects on aging and age-related diseases. Nutr. Rev. 2008, 66, 591–596. [Google Scholar] [CrossRef]
- Polivka, J.; Janku, F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol. Therap. 2014, 142, 164–175. [Google Scholar] [CrossRef]
- Alexander, G.E. Biology of Parkinson’s disease: Pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin. Neurosci. 2004, 6, 259–280. [Google Scholar]
- Miller, R.L.; James-Kracke, M.; Sun, G.Y.; Sun, A.Y. Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem. Res. 2009, 34, 55–65. [Google Scholar] [CrossRef]
- Miller, R.L.; Sun, G.Y.; Sun, A.Y. Cytotoxicity of paraquat in microglial cells: Involvement of PKCδ- and ERK1/2-dependent NADPH oxidase. Brain Res. 2007, 1167, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, O.; Mandel, S.; Amit, T.; Youdim, M.B. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J. Nutr. Biochem. 2004, 15, 506–516. [Google Scholar] [CrossRef]
- Mercer, L.D.; Kelly, B.L.; Horne, M.K.; Beart, P.M. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: Investigations in primary rat mesencephalic cultures. Biochem. Pharm. 2005, 69, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Suzuki, N.; Taniguchi, S.; Oikawa, T.; Nonaka, T.; Iwatsubo, T.; Hisanaga, S.; Goedert, M.; Hasegawa, M. Small molecule inhibitors of alpha-synuclein filament assembly. Biochemistry 2006, 45, 6085–6094. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, S.; Coley, N.; Lovestone, S.; Aisen, P.S.; Vellas, B. Prevention of sporadic Alzheimer’s disease: Lessons learned from clinical trials and future directions. Lancet Neurol. 2015, 14, 926–944. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [Green Version]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J. Alzheimers Dis. 2001, 3, 75–80. [Google Scholar] [CrossRef]
- Snyder, E.M.; Nong, Y.; Almeida, C.G.; Paul, S.; Moran, T.; Choi, E.Y.; Nairn, A.C.; Salter, M.W.; Lombroso, P.J.; Gouras, G.K.; et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005, 8, 1051–1058. [Google Scholar] [CrossRef]
- Flanagan, E.; Müller, M.; Hornberger, M.; Vauzour, D. Impact of flavonoids on cellular and molecular mechanisms underlying age-related cognitive decline and neurodegeneration. Curr. Nutr. Rep. 2018, 7, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Li, Y.H.; Xu, Y.; Li, Y.B.; Wu, H.L.; Guo, H.; Zhang, J.Z.; Zhang, J.J.; Pan, X.Y.; Li, X.J. Curcumin produces neuroprotective effects via activating brain-derived neurotrophic factor/TrkB-dependent MAPK and PI-3K cascades in rodent cortical neurons. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 147–153. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Xu, Y.; Lian, Y.; Xie, N.; Wu, T.; Zhang, H.; Sun, L.; Zhang, R.; Wang, Z. Curcumin improves amyloid β-peptide (1–42) induced spatial memory deficits through BDNF-ERK signaling pathway. PLoS ONE 2015, 10, e0131525. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Dai, X.; Xiao, N.; Wu, X.; Wei, Z.; Fang, W.; Zhu, Y.; Zhang, J.; Chen, X. Curcumin ameliorates memory decline via inhibiting BACE1 expression and β-amyloid pathology in 5 × FAD transgenic mice. Mol. Neurobiol. 2017, 54, 1967–1977. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Li, W.F.; Li, F.; Zhang, Z.; Dai, Y.D.; Xu, A.L.; Qi, C.; Gao, J.M.; Gao, J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem. Biophys. Res. Commun. 2013, 435, 597–602. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Yang, J.; Wei, Y.; Li, J. Effects of piceatannol and pterostilbene against β-amyloid-induced apoptosis on the PI3K/Akt/Bad signaling pathway in PC12 cells. Food Funct. 2016, 7, 1014–1023. [Google Scholar] [CrossRef]
- Donmez, G.; Outeiro, T.F. SIRT1 and SIRT2: Emerging targets in neurodegeneration. EMBO Mol. Med. 2013, 5, 344–352. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Pinto, J.T.; Xu, H.; Chen, H.L.; Beal, M.F.; Gibson, G.E. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem. Int. 2009, 54, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Zhang, Y.; Li, J.; Zhang, C. Resveratrol ameliorates spatial learning memory impairment induced by Aβ. Neuroscience 2017, 344, 39–47. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer’s transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef]
- Evin, G.; Barakat, A.; Masters, C.L. BACE: Therapeutic target and potential biomarker for Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2010, 42, 1923–1926. [Google Scholar] [CrossRef]
- Ortiz-López, L.; Márquez-Valadez, B.; Gómez-Sánchez, A.; Silva-Lucero, M.D.; Torres-Pérez, M.; Téllez-Ballesteros, R.I.; Ichwan, M.; Meraz-Ríos, M.A.; Kempermann, G.; Ramírez-Rodríguez, G.B. Green tea compound epigallo-catechin-3-gallate (EGCG) increases neuronal survival in adult hippocampal neurogenesis in vivo and in vitro. Neuroscience 2016, 322, 208–220. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.; Li, J.; Sun, Y.; Hasimu, H.; Liu, R.; Zhang, T. Quercetin protects human brain microvascular endothelial cells from fibrillar β-amyloid1–40-induced toxicity. Acta Pharm. Sin. B 2015, 5, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hu, J.; Zhong, L.; Wang, N.; Yang, L.; Liu, C.C.; Li, H.; Wang, X.; Zhou, Y.; Zhang, Y.; et al. Quercetin stabilizes apolipoprotein E and reduces brain Aβ levels in amyloid model mice. Neuropharmacology 2016, 108, 179–192. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Liu, Q.; Yu, Q. Quercetin enrich diet during the early-middle not middle-late stage of Alzheimer’s disease ameliorates cognitive dysfunction. Am. J. Transl. Res. 2018, 10, 1237–1246. [Google Scholar]
- Kong, Y.; Li, K.; Fu, T.; Wan, C.; Zhang, D.; Song, H.; Zhang, Y.; Liu, N.; Gan, Z.; Yuan, L. Quercetin ameliorates Aβ toxicity in Drosophila AD model by modulating cell cycle-related protein expression. Oncotarget 2016, 7, 67716–67731. [Google Scholar] [CrossRef] [Green Version]
- Cui, Q.; Li, X.; Zhu, H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol. Med. Rep. 2016, 13, 1381–1388. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Li, Q.; Wang, X.; Yu, S.; Li, L.; Wu, X.; Chen, Y.; Zhao, J.; Zhao, Y. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS ONE 2013, 8, e59843. [Google Scholar] [CrossRef] [Green Version]
- Ramkumar, M.; Rajasankar, S.; Swaminathan Johnson, W.M.; Prabu, K.; Venkatesh Gobi, V. Demethoxycurcumin ameliorates rotenone-induced toxicity in rats. Front. Biosci. 2019, 11, 1–11. [Google Scholar]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.L.; Ju, B.; Zhang, Y.Z.; Yin, H.L.; Liu, Y.J.; Wang, S.S.; Zeng, Z.L.; Yang, X.P.; Wang, H.T.; Li, J.F. Protective Effect of Curcumin Against Oxidative Stress-Induced Injury in Rats with Parkinson’s Disease Through the Wnt/ β-Catenin Signaling Pathway. Cell. Physiol. Biochem. 2017, 43, 2226–2241. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; He, X.; Wang, Y.; Gao, S.; Ren, L.; Shi, Y. Curcumin exerts anti-inflammatory and antioxidative properties in 1-methyl-4-phenylpyridinium ion (MPP(+))-stimulated mesencephalic astrocytes by interference with TLR4 and downstream signaling pathway. Cell Stress Chaperones 2016, 21, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Potdar, S.; Parmar, M.S.; Ray, S.D.; Cavanaugh, J.E. Protective effects of the resveratrol analog piceid in dopaminergic SH-SY5Y cells. Arch. Toxicol. 2018, 92, 669–677. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, W.; Lu, F.; Gao, L.; Gao, G. Resveratrol attenuates MPP. Neurosci. Lett. 2017, 637, 50–56. [Google Scholar] [CrossRef]
- Gaballah, H.H.; Zakaria, S.S.; Elbatsh, M.M.; Tahoon, N.M. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chem. Biol. Interact. 2016, 251, 10–16. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Madathil, S.K.; Pandey, M.; Haobam, R.; Rajamma, U.; Mohanakumar, K.P. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience 2013, 236, 136–148. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, C.S.; Kim, D.J.; Cho, M.H.; Jin, B.K.; Pie, J.E.; Chung, W.G. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology 2002, 23, 367–374. [Google Scholar] [CrossRef]
- Levites, Y.; Weinreb, O.; Maor, G.; Youdim, M.B.; Mandel, S. Green tea polyphenol(−)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 2001, 78, 1073–1082. [Google Scholar] [CrossRef]
- Walker, F.O. Huntington’s Disease. Semin. Neurol. 2007, 27, 143–150. [Google Scholar] [CrossRef]
- Strong, T.V.; Tagle, D.A.; Valdes, J.M.; Elmer, L.W.; Boehm, K.; Swaroop, M.; Kaatz, K.W.; Collins, F.S.; Albin, R.L. Widespread expression of the human and rat Huntington’s disease gene in brain and nonneural tissues. Nat. Genet. 1993, 5, 259–265. [Google Scholar] [CrossRef]
- Sciacca, S.; Favellato, M.; Madonna, M.; Metro, D.; Marano, M.; Squitieri, F. Early enteric neuron dysfunction in mouse and human Huntington’s disease. Parkinsonism Relat. Disord. 2017, 34, 73–74. [Google Scholar] [CrossRef]
- Siddiqui, A.; Rivera-Sánchez, S.; Castro, M.e.R.; Acevedo-Torres, K.; Rane, A.; Torres-Ramos, C.A.; Nicholls, D.G.; Andersen, J.K.; Ayala-Torres, S. Mitochondrial DNA damage is associated with reduced mitochondrial bioenergetics in Huntington’s disease. Free Radic. Biol. Med. 2012, 53, 1478–1488. [Google Scholar] [CrossRef] [Green Version]
- Xun, Z.; Rivera-Sánchez, S.; Ayala-Peña, S.; Lim, J.; Budworth, H.; Skoda, E.M.; Robbins, P.D.; Niedernhofer, L.J.; Wipf, P.; McMurray, C.T. Targeting of XJB-5–131 to mitochondria suppresses oxidative DNA damage and motor decline in a mouse model of Huntington’s disease. Cell Rep. 2012, 2, 1137–1142. [Google Scholar] [CrossRef] [Green Version]
- Naia, L.; Rosenstock, T.R.; Oliveira, A.M.; Oliveira-Sousa, S.I.; Caldeira, G.L.; Carmo, C.; Laço, M.N.; Hayden, M.R.; Oliveira, C.R.; Rego, A.C. Comparative mitochondrial-based protective effects of resveratrol and nicotinamide in Huntington’s disease models. Mol. Neurobiol. 2017, 54, 5385–5399. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. Neuromol. Med. 2014, 16, 106–118. [Google Scholar] [CrossRef]
- Chongtham, A.; Agrawal, N. Curcumin modulates cell death and is protective in Huntington’s disease model. Sci. Rep. 2016, 6, 18736. [Google Scholar] [CrossRef] [Green Version]
- Elifani, F.; Amico, E.; Pepe, G.; Capocci, L.; Castaldo, S.; Rosa, P.; Montano, E.; Pollice, A.; Madonna, M.; Filosa, S.; et al. Curcumin dietary supplementation ameliorates disease phenotype in an animal model of Huntington’s disease. Hum. Mol. Genet. 2019, 28, 4012–4021. [Google Scholar] [CrossRef]
- Sandhir, R.; Mehrotra, A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: Implications in Huntington’s disease. Biochim. Biophys. Acta 2013, 1832, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, J.; Singh, R.; Dutta, D.; Naskar, A.; Rajamma, U.; Mohanakumar, K.P. Quercetin improves behavioral deficiencies, restores astrocytes and microglia, and reduces serotonin metabolism in 3-nitropropionic acid-induced rat model of Huntington’s disease. CNS Neurosci. 2014, 20, 10–19. [Google Scholar] [CrossRef]
- Jellinger, K.A. The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol. 2007, 113, 349–388. [Google Scholar] [CrossRef]
- Molino, S.; Dossena, M.; Buonocore, D.; Ferrari, F.; Venturini, L.; Ricevuti, G.; Verri, M. Polyphenols in dementia: From molecular basis to clinical trials. Life Sci. 2016, 161, 69–77. [Google Scholar] [CrossRef]
- Awasthi, H.; Tota, S.; Hanif, K.; Nath, C.; Shukla, R. Protective effect of curcumin against intracerebral streptozotocin induced impairment in memory and cerebral blood flow. Life Sci. 2010, 86, 87–94. [Google Scholar] [CrossRef]
- Anastácio, J.R.; Netto, C.A.; Castro, C.C.; Sanches, E.F.; Ferreira, D.C.; Noschang, C.; Krolow, R.; Dalmaz, C.; Pagnussat, A. Resveratrol treatment has neuroprotective effects and prevents cognitive impairment after chronic cerebral hypoperfusion. Neurol. Res. 2014, 36, 627–633. [Google Scholar] [CrossRef]
- Shen, D.; Tian, X.; Sang, W.; Song, R. Effect of melatonin and resveratrol against memory impairment and hippocampal damage in a rat model of vascular dementia. Neuroimmunomodulation 2016, 23, 318–331. [Google Scholar] [CrossRef]
- Tota, S.; Awasthi, H.; Kamat, P.K.; Nath, C.; Hanif, K. Protective effect of quercetin against intracerebral streptozotocin induced reduction in cerebral blood flow and impairment of memory in mice. Behav. Brain Res. 2010, 209, 73–79. [Google Scholar] [CrossRef]
- Jayaraj, R.; Elangovan, N.; Manigandan, K.; Singh, S.; Shukla, S. CNB-001 a novel curcumin derivative, guards dopamine neurons in MPTP model of Parkinson’s disease. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [Green Version]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Aura, A. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Deprez, S.; Mila, I.; Huneau, J.F.; Tome, D.; Scalbert, A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid. Redox. Signal. 2001, 3, 957–967. [Google Scholar] [CrossRef]
- Kang, S.M.; Lee, S.H.; Heo, S.J.; Kim, K.N.; Jeon, Y.J. Evaluation of antioxidant properties of a new compound, pyrogallol-phloroglucinol-6,6’-bieckol isolated from brown algae, Ecklonia cava. Nutr. Res. Pract. 2011, 5, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Fang, Y. The three catecholics benserazide, catechol and pyrogallol are GPR35 agonists. Pharmaceuticals 2013, 6, 500–509. [Google Scholar] [CrossRef]
- Di Giovanni, S.; Eleuteri, S.; Paleologou, K.E.; Yin, G.; Zweckstetter, M.; Carrupt, P.A.; Lashuel, H.A. Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of alpha-synuclein and beta-amyloid and protect against amyloid-induced toxicity. J. Biol. Chem. 2010, 285, 14941–14954. [Google Scholar] [CrossRef] [Green Version]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, gut microbiota, and neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basholli-Salihu, M.; Schuster, R.; Mulla, D.; Praznik, W.; Viernstein, H.; Mueller, M. Bioconversion of piceid to resveratrol by selected probiotic cell extracts. Bioprocess. Biosyst. Eng. 2016, 39, 1879–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Blaut, M.; Schoefer, L.; Braune, A. Transformation of flavonoids by intestinal microorganisms. Int. J. Vitam. Nutr. Res. 2003, 73, 79–87. [Google Scholar] [CrossRef]
- Zheng, C.J.; Liu, R.; Xue, B.; Luo, J.; Gao, L.; Wang, Y.; Ou, S.; Li, S.; Peng, X. Impact and consequences of polyphenols and fructooligosaccharide interplay on gut microbiota in rats. Food Funct. 2017, 8, 1925–1932. [Google Scholar] [CrossRef]
- Angelino, D.; Carregosa, D.; Domenech-Coca, C.; Savi, M.; Figueira, I.; Brindani, N.; Jang, S.; Lakshman, S.; Molokin, A.; Urban, J.F.; et al. 5-(hydroxyphenyl)-γ-valerolactone-sulfate, a key microbial metabolite of flavan-3-ols, is able to reach the brain: Evidence from different in. Nutrients 2019, 11, 2678. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.L.; Kirk, R.D.; DaSilva, N.A.; Ma, H.; Seeram, N.P.; Bertin, M.J. Polyphenol microbial metabolites exhibit gut and blood-brain barrier permeability and protect murine microglia against LPS-induced inflammation. Metabolites 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural. Regen. Res. 2018, 13, 2055–2059. [Google Scholar]
- Bird, J.K.; Raederstorff, D.; Weber, P.; Steinert, R.E. Cardiovascular and antiobesity effects of resveratrol mediated through the gut microbiota. Adv. Nutr. 2017, 8, 839–849. [Google Scholar] [CrossRef]
- Arakawa, H.; Maeda, M.; Okubo, S.; Shimamura, T. Role of hydrogen peroxide in bactericidal action of catechin. Biol. Pharm. Bull. 2004, 27, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef] [Green Version]
- Yamakoshi, J.; Tokutake, S.; Kikuchi, M.; Kubota, Y.; Konishi, H.; Mitsuoka, T. Effect of Proanthocyanidin-Rich Extract from Grape Seeds on Human Fecal Flora and Fecal Odor. Microb. Ecol. Health Dis. 2001, 13, 25–31. [Google Scholar] [CrossRef]
- García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Brasili, E.; Hassimotto, N.M.A.; Del Chierico, F.; Marini, F.; Quagliariello, A.; Sciubba, F.; Miccheli, A.; Putignani, L.; Lajolo, F. Daily consumption of orange juice from Citrus sinensis L. Osbeck cv. Cara Cara and cv. Bahia differently affects gut microbiota profiling as unveiled by an integrated meta-omics approach. J. Agric. Food Chem. 2019, 67, 1381–1391. [Google Scholar] [CrossRef]
- Gerhardt, S.; Mohajeri, M.H. Changes of colonic bacterial composition in Parkinson’s disease and other neurodegenerative diseases. Nutrients 2018, 10, 708. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.F.; Shen, Y.Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res. Rev. 2018, 45, 53–61. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [Green Version]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011, 33, 574–581. [Google Scholar] [CrossRef]
- Nzakizwanayo, J.; Dedi, C.; Standen, G.; Macfarlane, W.M.; Patel, B.A.; Jones, B.V. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Sci. Rep. 2015, 5, 17324. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Yunes, R.A.; Poluektova, E.U.; Dyachkova, M.S.; Klimina, K.M.; Kovtun, A.S.; Averina, O.V.; Orlova, V.S.; Danilenko, V.N. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 2016, 42, 197–204. [Google Scholar] [CrossRef]



| Polyphenol | Signaling Pathway | References |
|---|---|---|
| Resveratrol | SIRT1/PGC-1 | [52,55] |
| PI3K/Akt | ||
| Curcumin | AMPK/NF-kB | [56,57,58] |
| PI3K/Akt/GSK-3β | ||
| Quercetin | MAPK/AKT/ PI3K | [59] |
| ERK/CREB | ||
| Catechins (EGCG) | PKC/MAPK/PI3K/Akt | [60,61,62] |
| MEK/ERK1/2 |
| Pathology | Type of Study | Polyphenols (dose) | Time | Effect | References |
|---|---|---|---|---|---|
| Alzheimer’s disease | mice | grape extract (5–20 μM) | 5 months | Inhibition of Aβ oligomerization | [3] |
| Parkinson’s disease | neuroblastoma cell line | caffeic acid (10–100 μM) | 1 hour | Prevention of apoptotic cell death | [9] |
| Neurodegenerative disorders | neonatal mouse cerebellum cells | curcumin (1–50 μM) | 24 hours | Enhancement and repair of neural plasticity | [37] |
| Alzheimer’s disease | rats | curcumin (50–200 mg/kg) | 7 days | Improvement of cognitive deficits | [71,72] |
| CNS disorders | mice | Resveratrol (20 mg/kg) | 7 days | Regulation of pathway involved in CNS disorder and aging | [73] |
| Alzheimer’s disease | mice | Resveratrol (24 mg/kg) | 45 days | Anti-oxidant effect against beta-amyloid plaque formation | [76,77] |
| Alzheimer’s disease | mice | ECGC (50 mg/kg) | 6 months | Reduction in A-β deposition | [78] |
| Alzheimer’s disease | human brain microvascular endothelial cells | Quercetin (0.3–30 μmol/L) | 24 hours | Increase in cell viability and of antioxidant activity | [81] |
| Parkinson’s disease | primary rat mesencephalic cells | Catechin, quercetin (40 μM) | 48 hours | Protective effect on DA neurons under oxidative stress | [61,70] |
| Parkinson’s disease | rodent model | Curcumin 30 mg/kg) | 4 days | Neuroprotective actions (anti-inflammatory and anti-oxidative) | [88,91,115] |
| Parkinson- like disease | dopaminergic-like cells | Resveratrol (50–200 μM) | 12 hours | Neuroprotective effects by inhibiting apoptosis caused by oxidative stress | [92] |
| Parkinson’s disease | rats | Resveratrol (20 mg/kg) | 21 days | Prevention of neuronal death | [94,95] |
| Parkinson’s disease | rats | Quercetin (25–75 mg/kg) | 4 days | Neuroprotective effect observed in neurotoxin-induced Parkinsonism | [95] |
| Parkinson’s disease | mice | ECGC (20 mg/kg) | 5 days | Preventive effects on NOS | [96,97] |
| Huntington’s disease | mice | ECGC (1 mg/kg) | 28 days | Improvement of gene transcription associated to mitochondrial function | [103] |
| Huntington’s disease | rats | Curcumin (40 mg/kg) | 7 days | Amelioration of mitochondrial dysfunctions | [104,105] |
| Huntington’s disease | mice | Curcumin (20–40 mg/kg) | 7 days | Alleviation of debilitating symptoms associated with the disease | [106] |
| Huntington’s disease | rats | Quercetin (25–50 mg/kg) | 4 days | Potential use for inflammatory damages | [107,108] |
| Memory and cerebral blood flow | mice | Curcumin (10–50 mg/kg) | 21 days | Beneficial effects of oxidative stress associated with neurodegenerative disorders | [111] |
| Dementia | rats | Resveratrol (10–20 mg/kg) | 4 days | Neurorestorative effects | [113] |
| Memory dysfunction | mice | Quercetin (2.5–10 mg/kg) | 21 days | Protective toward off dementia and neurodegenerative disorders | [114] |
| Polyphenols | Bacteria | References | |
|---|---|---|---|
| Catechin and epicatechin | + | Clostridium coccoides–Eubacterium rectale | [134] |
| + | E. coli | ||
| − | Clostridium histolyticum | ||
| Proanthocyanidin | + | Bifidobacteria | [135] |
| Pomegranate extract | + | Odonbacter | [136] |
| + | Faecalibacterium | ||
| + | Butyricicoccus | ||
| + | Butyricimonas | ||
| + | Bacteroides | ||
| − | Parvimonas | ||
| − | Metanobrevibacter | ||
| − | Metanosphaera | ||
| Cocoa flavonols | + | Bifidobacterium | [137] |
| − | Lactobacillus | ||
| − | Clostridia | ||
| Red wine | + | Enterococcus | [138] |
| + | Prevotella | ||
| + | Bacteroides | ||
| + | Bifidobacterium | ||
| + | Enterococcus | ||
| + | Bacteroides uniformis | ||
| + | Eggerthella lenta | ||
| + | Blautia coccoides | ||
| Orange juice | + | Mogibacteriaceae | [139] |
| + | Tissierellaceae | ||
| + | Veillonellaceae | ||
| + | Odoribacteraceae | ||
| + | Ruminococcaceae |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 2564. https://doi.org/10.3390/ijms21072564
Di Meo F, Valentino A, Petillo O, Peluso G, Filosa S, Crispi S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. International Journal of Molecular Sciences. 2020; 21(7):2564. https://doi.org/10.3390/ijms21072564
Chicago/Turabian StyleDi Meo, Francesco, Anna Valentino, Orsolina Petillo, Gianfranco Peluso, Stefania Filosa, and Stefania Crispi. 2020. "Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration" International Journal of Molecular Sciences 21, no. 7: 2564. https://doi.org/10.3390/ijms21072564
APA StyleDi Meo, F., Valentino, A., Petillo, O., Peluso, G., Filosa, S., & Crispi, S. (2020). Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. International Journal of Molecular Sciences, 21(7), 2564. https://doi.org/10.3390/ijms21072564