Aedes albopictus Autophagy-Related Gene 8 (AaAtg8) Is Required to Confer Anti-Bacterial Gut Immunity
Abstract
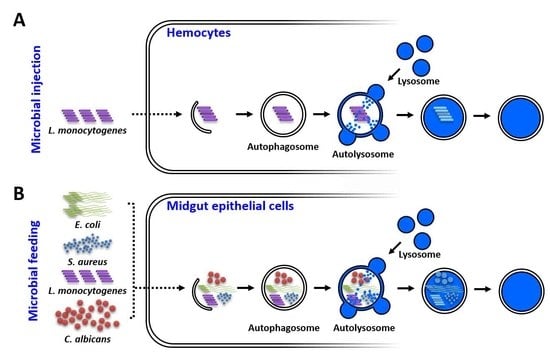
:1. Introduction
2. Results
2.1. Sequence Analysis of AaAtg8
2.2. Developmental and Tissue Distribution of AaAtg8 at mRNA Level
2.3. Expression of AaAtg8 mRNA after Immune Challenge
2.4. Effects of AaAtg8 Gene Silencing on the Survivability of Ae. Albopictus Mosquitoes
3. Discussion
4. Materials and Methods
4.1. Mosquito Rearing
4.2. Microbial Cultures and Infection of Mosquitoes
4.3. Identification and in Silico Analysis of AaAtg8
4.4. Expression and Induction Patterns of AaAtg8
4.5. dsRNA Synthesis and AaAtg8 Silencing in Female Mosquitoes
4.6. Effect of AaAtg8 Silencing on Survival of Mosquitoes after Inoculation with Microorganisms
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McPhee, C.K.; Baehrecke, E.H. Autophagy in drosophila melanogaster. Biochim. Biophys Acta 2009, 1793, 1452–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravanan, P.; Srikumar, I.F.; Talwar, P. Autophagy: The spotlight for cellular stress responses. Life Sci. 2017, 188, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Erustes, A.G.; Costa, A.J.; Nascimento, A.C.; Bincoletto, C.; Ureshino, R.P.; Pereira, G.J.S.; Smaili, S.S. Autophagy and intermittent fasting: The connection for cancer therapy? Clin. (Sao Paulo) 2018, 73, e814s. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.M.; Yoshimori, T.; Jo, E.K. Autophagy and bacterial infectious diseases. Exp. Mol. Med. 2012, 44, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tindwa, H.; Jo, Y.H.; Patnaik, B.B.; Lee, Y.S.; Kang, S.S.; Han, Y.S. Molecular cloning and characterization of autophagy-related gene tmatg8 in listeria-invaded hemocytes of tenebrio molitor. Dev. Comp. Immunol. 2015, 51, 88–98. [Google Scholar] [CrossRef]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef] [Green Version]
- Reggiori, F.; Klionsky, D.J. Autophagic processes in yeast: Mechanism, machinery and regulation. Genetics 2013, 194, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Motley, A.M.; Nuttall, J.M.; Hettema, E.H. Pex3-anchored atg36 tags peroxisomes for degradation in saccharomyces cerevisiae. EMBO J. 2012, 31, 2852–2868. [Google Scholar] [CrossRef] [Green Version]
- Kamada, Y.; Yoshino, K.; Kondo, C.; Kawamata, T.; Oshiro, N.; Yonezawa, K.; Ohsumi, Y. Tor directly controls the atg1 kinase complex to regulate autophagy. Mol. Cell Biol. 2010, 30, 1049–1058. [Google Scholar] [CrossRef] [Green Version]
- Nair, U.; Cao, Y.; Xie, Z.; Klionsky, D.J. Roles of the lipid-binding motifs of atg18 and atg21 in the cytoplasm to vacuole targeting pathway and autophagy. J. Biol. Chem. 2010, 285, 11476–11488. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Kakuta, S.; Watanabe, T.M.; Kitamura, A.; Sekito, T.; Kondo-Kakuta, C.; Ichikawa, R.; Kinjo, M.; Ohsumi, Y. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J. Cell Biol. 2012, 198, 219–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell Biol. 2007, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.N.; Fujioka, Y.; Hanada, T.; Ohsumi, Y.; Inagaki, F. Structure of the atg12-atg5 conjugate reveals a platform for stimulating atg8-pe conjugation. EMBO Rep. 2013, 14, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Otomo, C.; Metlagel, Z.; Takaesu, G.; Otomo, T. Structure of the human atg12~atg5 conjugate required for lc3 lipidation in autophagy. Nat. Struct Mol. Biol. 2013, 20, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Nair, U.; Klionsky, D.J. Atg8 controls phagophore expansion during autophagosome formation. Mol. Biol. Cell 2008, 19, 3290–3298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatogawa, H.; Ichimura, Y.; Ohsumi, Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell 2007, 130, 165–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, N.N.; Kumeta, H.; Nakatogawa, H.; Satoo, K.; Adachi, W.; Ishii, J.; Fujioka, Y.; Ohsumi, Y.; Inagaki, F. Structural basis of target recognition by atg8/lc3 during selective autophagy. Genes Cells 2008, 13, 1211–1218. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, X.; Teng, Y.B.; Hu, H.X.; Li, W.F. Structure of autophagy-related protein atg8 from the silkworm bombyx mori. Acta Crystallogr Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 787–790. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lu, H.; Ai, H.; Peng, R.; Yang, Y.; Li, A.; Hong, H.; Peng, J.; Liu, K. Distribution, cleavage and lipidation of atg8 fusion proteins in spodoptera litura sl-hp cells. PLoS ONE 2014, 9, e96059. [Google Scholar] [CrossRef]
- Khoa, D.B.; Takeda, M. Expression of autophagy 8 (atg8) and its role in the midgut and other organs of the greater wax moth, galleria mellonella, during metamorphic remodelling and under starvation. Insect Mol. Biol 2012, 21, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Bryant, B.; Raikhel, A.S. Programmed autophagy in the fat body of aedes aegypti is required to maintain egg maturation cycles. PLoS ONE 2011, 6, e25502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellner, R.; De la Concepcion, J.C.; Maqbool, A.; Kamoun, S.; Dagdas, Y.F. Atg8 expansion: A driver of selective autophagy diversification? Trends Plant. Sci. 2017, 22, 204–214. [Google Scholar] [CrossRef]
- Shpilka, T.; Weidberg, H.; Pietrokovski, S.; Elazar, Z. Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. 2011, 12. [Google Scholar] [CrossRef] [PubMed]
- King, N.; Westbrook, M.J.; Young, S.L.; Kuo, A.; Abedin, M.; Chapman, J.; Fairclough, S.; Hellsten, U.; Isogai, Y.; Letunic, I.; et al. The genome of the choanoflagellate monosiga brevicollis and the origin of metazoans. Nature 2008, 451, 783–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, M.; Simakov, O.; Chapman, J.; Fahey, B.; Gauthier, M.E.; Mitros, T.; Richards, G.S.; Conaco, C.; Dacre, M.; Hellsten, U.; et al. The amphimedon queenslandica genome and the evolution of animal complexity. Nature 2010, 466, 720–726. [Google Scholar] [CrossRef]
- Brackney, D.E.; Correa, M.A. A limited role for autophagy during arbovirus infection of mosquito cells. bioRxiv 2019, 760728. [Google Scholar]
- Kumeta, H.; Watanabe, M.; Nakatogawa, H.; Yamaguchi, M.; Ogura, K.; Adachi, W.; Fujioka, Y.; Noda, N.N.; Ohsumi, Y.; Inagaki, F. The nmr structure of the autophagy-related protein atg8. J. Biomol. NMR 2010, 47, 237–241. [Google Scholar] [CrossRef]
- Bavro, V.N.; Sola, M.; Bracher, A.; Kneussel, M.; Betz, H.; Weissenhorn, W. Crystal structure of the gaba(a)-receptor-associated protein, gabarap. EMBO Rep. 2002, 3, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Nakatogawa, H.; Ohbayashi, S.; Sakoh-Nakatogawa, M.; Kakuta, S.; Suzuki, S.W.; Kirisako, H.; Kondo-Kakuta, C.; Noda, N.N.; Yamamoto, H.; Ohsumi, Y. The autophagy-related protein kinase atg1 interacts with the ubiquitin-like protein atg8 via the atg8 family interacting motif to facilitate autophagosome formation. J. Biol. Chem. 2012, 287, 28503–28507. [Google Scholar] [CrossRef] [Green Version]
- McEwan, D.G.; Popovic, D.; Gubas, A.; Terawaki, S.; Suzuki, H.; Stadel, D.; Coxon, F.P.; Miranda de Stegmann, D.; Bhogaraju, S.; Maddi, K.; et al. Plekhm1 regulates autophagosome-lysosome fusion through hops complex and lc3/gabarap proteins. Mol. Cell 2015, 57, 39–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichimura, Y.; Kirisako, T.; Takao, T.; Satomi, Y.; Shimonishi, Y.; Ishihara, N.; Mizushima, N.; Tanida, I.; Kominami, E.; Ohsumi, M.; et al. A ubiquitin-like system mediates protein lipidation. Nature 2000, 408, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Amar, N.; Lustig, G.; Ichimura, Y.; Ohsumi, Y.; Elazar, Z. Two newly identified sites in the ubiquitin-like protein atg8 are essential for autophagy. EMBO Rep. 2006, 7, 635–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koopmann, R.; Muhammad, K.; Perbandt, M.; Betzel, C.; Duszenko, M. Trypanosoma brucei atg8: Structural insights into autophagic-like mechanisms in protozoa. Autophagy 2009, 5, 1085–1091. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Wu, Y.; Fu, H.; Ge, X.; You, H.; Wu, X. Identification and characterization of four autophagy-related genes that are expressed in response to hypoxia in the brain of the oriental river prawn (macrobrachium nipponense). Int. J. Mol. Sci 2019, 20, 1856. [Google Scholar] [CrossRef] [Green Version]
- Boya, P.; Reggiori, F.; Codogno, P. Emerging regulation and functions of autophagy. Nat. Cell Biol. 2013, 15, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Abreu, S.; Kriegenburg, F.; Gomez-Sanchez, R.; Mari, M.; Sanchez-Wandelmer, J.; Skytte Rasmussen, M.; Soares Guimaraes, R.; Zens, B.; Schuschnig, M.; Hardenberg, R.; et al. Conserved atg8 recognition sites mediate atg4 association with autophagosomal membranes and atg8 deconjugation. EMBO Rep. 2017, 18, 765–780. [Google Scholar] [CrossRef] [Green Version]
- Denton, D.; Shravage, B.; Simin, R.; Mills, K.; Berry, D.L.; Baehrecke, E.H.; Kumar, S. Autophagy, not apoptosis, is essential for midgut cell death in drosophila. Curr. Biol. 2009, 19, 1741–1746. [Google Scholar] [CrossRef] [Green Version]
- Nezis, I.P.; Lamark, T.; Velentzas, A.D.; Rusten, T.E.; Bjorkoy, G.; Johansen, T.; Papassideri, I.S.; Stravopodis, D.J.; Margaritis, L.H.; Stenmark, H.; et al. Cell death during drosophila melanogaster early oogenesis is mediated through autophagy. Autophagy 2009, 5, 298–302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hu, Z.Y.; Li, W.F.; Li, Q.R.; Deng, X.J.; Yang, W.Y.; Cao, Y.; Zhou, C.Z. Systematic cloning and analysis of autophagy-related genes from the silkworm bombyx mori. BMC Mol. Biol. 2009, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Hakim, R.S.; Baldwin, K.; Smagghe, G. Regulation of midgut growth, development, and metamorphosis. Annu. Rev. Entomol. 2010, 55, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. Lc3, a mammalian homologue of yeast apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.C.; Schuldiner, O.; Neufeld, T.P. Role and regulation of starvation-induced autophagy in the drosophila fat body. Dev. Cell 2004, 7, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, G.; Cheng, M.I.; Chen, C.; Nguyen, B.N.; Whiteley, A.T.; Kianian, S.; Cox, J.S.; Green, D.R.; McDonald, K.L.; Portnoy, D.A. Listeria monocytogenes triggers noncanonical autophagy upon phagocytosis, but avoids subsequent growth-restricting xenophagy. Proc. Natl. Acad. Sci. USA 2018, 115, E210–E217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, M.D.S.; Ribeiro, R.M.; Travassos, L.H. Autophagy and its interaction with intracellular bacterial pathogens. Front. Immunol. 2018, 9, 935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaughnessy, L.M.; Hoppe, A.D.; Christensen, K.A.; Swanson, J.A. Membrane perforations inhibit lysosome fusion by altering ph and calcium in listeria monocytogenes vacuoles. Cell Microbiol 2006, 8, 781–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, R.; Shaughnessy, L.; Loessner, M.J.; Alberti-Segui, C.; Higgins, D.E.; Swanson, J.A. Cytolysin-dependent delay of vacuole maturation in macrophages infected with listeria monocytogenes. Cell Microbiol 2006, 8, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.L.; Finkel, S.E.; Tower, J. Conditional inhibition of autophagy genes in adult drosophila impairs immunity without compromising longevity. Exp. Gerontol 2009, 44, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Yano, T.; Mita, S.; Ohmori, H.; Oshima, Y.; Fujimoto, Y.; Ueda, R.; Takada, H.; Goldman, W.E.; Fukase, K.; Silverman, N.; et al. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat. Immunol. 2008, 9, 908–916. [Google Scholar] [CrossRef] [Green Version]
- Tindwa, H.; Patnaik, B.B.; Kim, D.H.; Mun, S.; Jo, Y.H.; Lee, B.L.; Lee, Y.S.; Kim, N.J.; Han, Y.S. Cloning, characterization and effect of tmpgrp-le gene silencing on survival of tenebrio molitor against listeria monocytogenes infection. Int. J. Mol. Sci 2013, 14, 22462–22482. [Google Scholar] [CrossRef]
- Joyce, S.A.; Gahan, C.G.M. Molecular pathogenesis of listeria monocytogenes in the alternative model host galleria mellonella. Microbiol-Sgm 2010, 156, 3456–3468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchon, N.; Broderick, N.A.; Poidevin, M.; Pradervand, S.; Lemaitre, B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe 2009, 5, 200–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Lee, W.J. Role of duox in gut inflammation: Lessons from drosophila model of gut-microbiota interactions. Front. Cell Infect. Mi 2014, 3, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Liu, Y.; Wang, P.H.; Xiao, X.P. Mosquito defense strategies against viral infection. Trends Parasitol 2016, 32, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Webster, J.A.; Madzokere, E.T.; Stephenson, E.B.; Herrero, L.J. Mosquito antiviral defense mechanisms: A delicate balance between innate immunity and persistent viral infection. Parasite Vector 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal w and clustal x version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(t) (-delta delta c) method. Methods 2001, 25, 402–404. [Google Scholar] [CrossRef]







| Name | Primer Sequences |
|---|---|
| AaAtg8-cloning-452bp-Fw AaAtg8-cloning-452bp-Rv | 5′-TGTTTAGTGGATAACGCTCCTG-3′ 5′-TCCTTTTCTATACATATCACTCTCGTT-3′ |
| AaAtg8-qPCR-Fw AaAtg8-qPCR-Rv | 5′-TACAAGGAAGAACACCCCTTCG-3′ 5′-AATCTCCAATGCGAGCCTTG-3′ |
| AaAtg8-dsRNA-296bp-Fw AaAtg8-dsRNA-296bp-Rv | 5′-TAATACGACTCACTATAGGGT AAAGGCCGAGGGAGATAAAA-3′ 5′-TAATACGACTCACTATAGGGT GGTGTTCCTGGTACAGCGAG-3′ |
| EGFP-dsRNA-Fw EGFP-dsRNA-Rv | 5′-TAATACGACTCACTATAGGGT ACGTAAACGGCCACAAGTTC-3′ 5′-TAATACGACTCACTATAGGGT TGCTCAGGTAGTGTTGTCG-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.E.; Park, K.B.; Ko, H.J.; Keshavarz, M.; Bae, Y.M.; Kim, B.; Patnaik, B.B.; Jang, H.A.; Lee, Y.S.; Han, Y.S.; et al. Aedes albopictus Autophagy-Related Gene 8 (AaAtg8) Is Required to Confer Anti-Bacterial Gut Immunity. Int. J. Mol. Sci. 2020, 21, 2944. https://doi.org/10.3390/ijms21082944
Kim CE, Park KB, Ko HJ, Keshavarz M, Bae YM, Kim B, Patnaik BB, Jang HA, Lee YS, Han YS, et al. Aedes albopictus Autophagy-Related Gene 8 (AaAtg8) Is Required to Confer Anti-Bacterial Gut Immunity. International Journal of Molecular Sciences. 2020; 21(8):2944. https://doi.org/10.3390/ijms21082944
Chicago/Turabian StyleKim, Chang Eun, Ki Beom Park, Hye Jin Ko, Maryam Keshavarz, Young Min Bae, BoBae Kim, Bharat Bhusan Patnaik, Ho Am Jang, Yong Seok Lee, Yeon Soo Han, and et al. 2020. "Aedes albopictus Autophagy-Related Gene 8 (AaAtg8) Is Required to Confer Anti-Bacterial Gut Immunity" International Journal of Molecular Sciences 21, no. 8: 2944. https://doi.org/10.3390/ijms21082944
APA StyleKim, C. E., Park, K. B., Ko, H. J., Keshavarz, M., Bae, Y. M., Kim, B., Patnaik, B. B., Jang, H. A., Lee, Y. S., Han, Y. S., & Jo, Y. H. (2020). Aedes albopictus Autophagy-Related Gene 8 (AaAtg8) Is Required to Confer Anti-Bacterial Gut Immunity. International Journal of Molecular Sciences, 21(8), 2944. https://doi.org/10.3390/ijms21082944