Regenerative Rehabilitation for Stroke Recovery by Inducing Synergistic Effects of Cell Therapy and Neurorehabilitation on Motor Function: A Narrative Review of Pre-Clinical Studies
Abstract
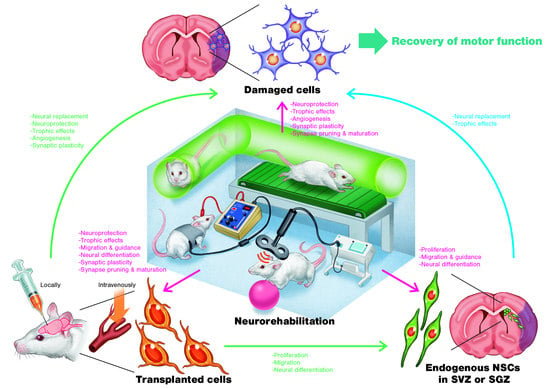
:1. Introduction
2. Cell Therapy for Stroke Recovery
3. Regenerative Rehabilitation
3.1. Regenerative Rehabilitation for Stroke Recovery
3.2. Effects of Combining Intravenous Cell Transplantation and Rehabilitation
3.3. Effects of Combining Local Cell Transplantation and Rehabilitation
4. Brain Stimulation for Stroke Recovery
4.1. Epidural Cortical Stimulation (CS)
4.2. Repetitive Transcranial Magnetic Stimulation (rTMS)
4.3. Effects of rTMS on Animal Models of Stroke
4.4. Transcranial Direct Current Stimulation (tDCS)
Effects of tDCS on Animal Models of Stroke
4.5. Therapeutic Effects of Combining Cell Transplantation and Brain Stimulation
5. Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| QOL | Quality of life |
| STEPS | Stem cell therapies as an emerging paradigm in stroke |
| MSCs | Mesenchymal stem cells |
| NSCs | Neural stem cells |
| NPCs | Neural progenitor cells |
| MCAO | Middle cerebral artery occlusion |
| SVZ | Subventricular zone |
| SGZ | Subgranular zone |
| EE | Enriched environment |
| rTMS | Repetitive transcranial magnetic stimulation |
| tDCS | Transcranial direct current stimulation |
| rADSCs | Rat adipose-derived stem cells |
| hADMSCs | Human adipose-tissue-derived mesenchymal stem cells |
| hASCs | Human adipose stem cells |
| FGF-2 | Fibroblast growth factor-2 |
| VCAM-1 | Vascular cell adhesion protein-1 |
| MMP-2 | Matrix metalloproteinase-2 |
| ANGPT-1 | Angiopoietin-1 |
| ANGPT-2 | Angiopoietin-2 |
| hESC | Human embryonic stem cell |
| CS | Epidural cortical stimulation |
| NIBS | Noninvasive brain stimulation |
| BDNF | Brain-derived neurotrophic factor |
| MAPK | Mitogen-activated Protein Kinase |
| cAMP | Cyclic adenosine monophosphate |
| MAP-2 | Microtubule-associated protein-2 |
| GAP-43 | Growth associated protein-43 |
| SDF-1α | Stromal cell-derived factor-1α |
| MCPTs | Multicenter pre-clinical trials |
References
- Gooch, C.L.; Pracht, E.; Borenstein, A.R. The burden of neurological disease in the United States: A summary report and call to action. Ann. Neurol. 2017, 81, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.J.; Finklestein, S.P.; Cramer, S.C. New directions in treatments targeting stroke recovery. Stroke 2018, 49, 3107–3114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Savitz, S.I.; Cramer, S.C.; Wechsler, L. Stem cells as an emerging paradigm in stroke 3: Enhancing the development of clinical trials. Stroke 2014, 45, 634–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boltze, J.; Modo, M.M.; Mays, R.W.; Taguchi, A.; Jolkkonen, J.; Savitz, S.I. Stem cells as an emerging paradigm in stroke 4: Advancing and accelerating preclinical research. Stroke 2019, 50, 3299–3306. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, F.; Russell, A. Regenerative rehabilitation: A call to action. J. Rehabil. Res. Dev. 2010, 47, xi–xv. [Google Scholar] [CrossRef]
- Lindvall, O.; Kokaia, Z. Stem cell research in stroke: How far from the clinic? Stroke 2011, 42, 2369–2375. [Google Scholar] [CrossRef] [Green Version]
- Daadi, M.M.; Hu, S.; Klausner, J.; Li, Z.; Sofilos, M.; Sun, G.; Wu, J.C.; Steinberg, G.K. Imaging neural stem cell graft-induced structural repair in stroke. Cell Transpl. 2013, 22, 881–892. [Google Scholar] [CrossRef] [Green Version]
- Horie, N.; Hiu, T.; Nagata, I. Stem cell transplantation enhances endogenous brain repair after experimental stroke. Neurol. Med. Chir. 2015, 55, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.; Lee, S.H.; Kim, S.U.; Yoon, B.W. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen. Res. 2016, 11, 298–304. [Google Scholar] [CrossRef]
- George, P.M.; Steinberg, G.K. Novel stroke therapeutics: Unraveling stroke pathophysiology and its impact on clinical treatments. Neuron 2015, 87, 297–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.Y.; Park, S.H.; Lee, S.U.; Choi, D.H.; Park, H.W.; Paek, S.H.; Shin, H.Y.; Kim, E.Y.; Park, S.P.; Lim, J.H. Effect of human embryonic stem cell-derived neuronal precursor cell transplantation into the cerebral infarct model of rat with exercise. Neurosci. Res. 2007, 58, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Tatarishvili, J.; Wood, J.; Koch, P.; Wattananit, S.; Mine, Y.; Monni, E.; Tornero, D.; Ahlenius, H.; Ladewig, J.; et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem. Cells 2012, 30, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Peron, S.; Droguerre, M.; Debarbieux, F.; Ballout, N.; Benoit-Marand, M.; Francheteau, M.; Brot, S.; Rougon, G.; Jaber, M.; Gaillard, A.A. Delay between motor cortex lesions and neuronal transplantation enhances graft integration and improves repair and recovery. J. Neurosci. 2017, 37, 1820–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroemer, P.; Patel, S.; Hope, A.; Oliveira, C.; Pollock, K.; Sinden, J. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil. Neural Repair 2009, 23, 895–909. [Google Scholar] [CrossRef] [Green Version]
- Kelly, S.; Bliss, T.M.; Shah, A.K.; Sun, G.H.; Ma, M.; Foo, W.C.; Masel, J.; Yenari, M.A.; Weissman, I.L.; Uchida, N.; et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 11839–11844. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.J.; Stroemer, R.P.; Gorenkova, N.; Nakajima, M.; Crum, W.R.; Tang, E.; Stevanato, L.; Sinden, J.D.; Modo, M. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem. Cells 2012, 30, 785–796. [Google Scholar] [CrossRef]
- Gaillard, A.; Prestoz, L.; Dumartin, B.; Cantereau, A.; Morel, F.; Roger, M.; Jaber, M. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat. Neurosci. 2007, 10, 1294–1299. [Google Scholar] [CrossRef]
- Tornero, D.; Tsupykov, O.; Granmo, M.; Rodriguez, C.; Gronning-Hansen, M.; Thelin, J.; Smozhanik, E.; Laterza, C.; Wattananit, S.; Ge, R.; et al. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain 2017, 140, 692–706. [Google Scholar] [CrossRef] [Green Version]
- Palma-Tortosa, S.; Tornero, D.; Gronning Hansen, M.; Monni, E.; Hajy, M.; Kartsivadze, S.; Aktay, S.; Tsupykov, O.; Parmar, M.; Deisseroth, K.; et al. Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 9094–9100. [Google Scholar] [CrossRef]
- Michelsen, K.A.; Acosta-Verdugo, S.; Benoit-Marand, M.; Espuny-Camacho, I.; Gaspard, N.; Saha, B.; Gaillard, A.; Vanderhaeghen, P. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron 2015, 85, 982–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrigno, M.; Busti, I.; Alia, C.; Pietrasanta, M.; Arisi, I.; D’Onofrio, M.; Caleo, M.; Cremisi, F. Neurons generated by mouse ESCs with Hippocampal or cortical identity display distinct projection patterns when co-transplanted in the adult brain. Stem. Cell Rep. 2018, 10, 1016–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.N.; Wang, Z.; Xu, T.Y.; Cheng, M.H.; Li, W.L.; Miao, C.Y. Cerebral organoids repair ischemic stroke brain injury. Transl. Stroke Res. 2019. [Epub ahead of print]. [Google Scholar] [CrossRef] [Green Version]
- Knoflach, M.; Matosevic, B.; Rucker, M.; Furtner, M.; Mair, A.; Wille, G.; Zangerle, A.; Werner, P.; Ferrari, J.; Schmidauer, C.; et al. Functional recovery after ischemic stroke—A matter of age: Data from the Austrian Stroke Unit Registry. Neurology 2012, 78, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Park, K.I.; Teng, Y.D.; Snyder, E.Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 2002, 20, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Modo, M.M.; Jolkkonen, J.; Zille, M.; Boltze, J. Future of animal modeling for poststroke tissue repair. Stroke 2018, 49, 1099–1106. [Google Scholar] [CrossRef]
- George, P.M.; Oh, B.; Dewi, R.; Hua, T.; Cai, L.; Levinson, A.; Liang, X.; Krajina, B.A.; Bliss, T.M.; Heilshorn, S.C.; et al. Engineered stem cell mimics to enhance stroke recovery. Biomaterials 2018, 178, 63–72. [Google Scholar] [CrossRef]
- Nicholls, F.J.; Ling, W.; Ferrauto, G.; Aime, S.; Modo, M. Simultaneous MR imaging for tissue engineering in a rat model of stroke. Sci. Rep. 2015, 5, 14597. [Google Scholar] [CrossRef] [Green Version]
- Modo, M. Bioscaffold-induced brain tissue regeneration. Front. Neurosci. 2019, 13, 1156. [Google Scholar] [CrossRef] [Green Version]
- Rando, T.A.; Ambrosio, F. Regenerative rehabilitation: Applied biophysics meets stem cell therapeutics. Cell Stem. Cell 2018, 22, 306–309. [Google Scholar] [CrossRef] [Green Version]
- Lo, A.C.; Guarino, P.D.; Richards, L.G.; Haselkorn, J.K.; Wittenberg, G.F.; Federman, D.G.; Ringer, R.J.; Wagner, T.H.; Krebs, H.I.; Volpe, B.T.; et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N. Engl. J. Med. 2010, 362, 1772–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klamroth-Marganska, V.; Blanco, J.; Campen, K.; Curt, A.; Dietz, V.; Ettlin, T.; Felder, M.; Fellinghauer, B.; Guidali, M.; Kollmar, A.; et al. Three-dimensional, task-specific robot therapy of the arm after stroke: A multicentre, parallel-group randomised trial. Lancet Neurol. 2014, 13, 159–166. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Langbroek-Amersfoort, A.C.; van Wegen, E.E.; Meskers, C.G.; Kwakkel, G. Effects of robot-assisted therapy for the upper limb after stroke. Neurorehabil. Neural Repair 2017, 31, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Modo, M. Exploring “Synergistic” effects of physical and cell therapy in stroke. In Proceedings of the 6th Annual International Symposium on Regenerative Rehabilitation, Pittsburgh, PA, USA, 1–3 November 2017. [Google Scholar]
- Hicks, A.U.; Hewlett, K.; Windle, V.; Chernenko, G.; Ploughman, M.; Jolkkonen, J.; Weiss, S.; Corbett, D. Enriched environment enhances transplanted subventricular zone stem cell migration and functional recovery after stroke. Neuroscience 2007, 146, 31–40. [Google Scholar] [CrossRef]
- Hicks, A.U.; MacLellan, C.L.; Chernenko, G.A.; Corbett, D. Long-term assessment of enriched housing and subventricular zone derived cell transplantation after focal ischemia in rats. Brain Res. 2008, 1231, 103–112. [Google Scholar] [CrossRef]
- Hicks, A.U.; Lappalainen, R.S.; Narkilahti, S.; Suuronen, R.; Corbett, D.; Sivenius, J.; Hovatta, O.; Jolkkonen, J. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: Cell survival and functional recovery. Eur. J. Neurosci. 2009, 29, 562–574. [Google Scholar] [CrossRef]
- Seo, J.H.; Kim, H.; Park, E.S.; Lee, J.E.; Kim, D.W.; Kim, H.O.; Im, S.H.; Yu, J.H.; Kim, J.Y.; Lee, M.Y.; et al. Environmental enrichment synergistically improves functional recovery by transplanted adipose stem cells in chronic hypoxic-ischemic brain injury. Cell Transpl. 2013, 22, 1553–1568. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Yuan, M.Z.; Cheng, L.; Lin, L.Z.; Du, H.W.; Chen, R.H.; Liu, N. Treadmill exercise enhances therapeutic potency of transplanted bone mesenchymal stem cells in cerebral ischemic rats via anti-apoptotic effects. BMC Neurosci. 2015, 16, 56. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.R.; Suh, H.; Yu, J.H.; Kim, H.H.; Seo, J.H.; Seo, C.H. Astroglial activation by an enriched environment after transplantation of mesenchymal stem cells enhances angiogenesis after hypoxic-ischemic brain injury. Int. J. Mol. Sci. 2016, 17, 1550. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y.; Sasaki, M.; Kataoka-Sasaki, Y.; Nakazaki, M.; Nagahama, H.; Suzuki, J.; Tateyama, D.; Oka, S.; Namioka, T.; Namioka, A.; et al. Synergic effects of rehabilitation and intravenous infusion of mesenchymal stem cells after stroke in rats. Phys. Ther. 2016, 96, 1791–1798. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Li, R.; Bi, S.; Li, Y.; Liu, L.; Jia, Y.L.; Han, P.; Gu, C.C.; Guo, X.Z.; Zhang, W.P.; et al. Combination of mild therapeutic hypothermia and adipose-derived stem cells for ischemic brain injury. Neural Regen. Res. 2018, 13, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Bakreen, A.; Juntunen, M.; Korhonen, P.; Oinonen, E.; Cui, L.; Myllyniemi, M.; Zhao, S.; Miettinen, S.; Jolkkonen, J. Combined adipose tissue-derived mesenchymal stem cell therapy and rehabilitation in experimental stroke. Front. Neurol. 2019, 10, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrossy, M.D.; Dunnett, S.B. The influence of environment and experience on neural grafts. Nat. Rev. Neurosci. 2001, 2, 871–879. [Google Scholar] [CrossRef]
- Dayan, E.; Censor, N.; Buch, E.R.; Sandrini, M.; Cohen, L.G. Noninvasive brain stimulation: From physiology to network dynamics and back. Nat. Neurosci. 2013, 16, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, W.; Abo, M.; Sasanuma, J.; Shimizu, M.; Okamoto, T.; Kimura, C.; Kakita, K.; Hara, H. Combination protocol of low-frequency rtms and intensive occupational therapy for post-stroke upper limb hemiparesis: A 6-year experience of more than 1700 Japanese patients. Transl. Stroke Res. 2016, 7, 172–179. [Google Scholar] [CrossRef]
- Boonzaier, J.; van Tilborg, G.A.F.; Neggers, S.F.W.; Dijkhuizen, R.M. Noninvasive brain stimulation to enhance functional recovery after stroke: Studies in animal models. Neurorehabil. Neural Repair 2018, 32, 927–940. [Google Scholar] [CrossRef]
- Fisicaro, F.; Lanza, G.; Grasso, A.A.; Pennisi, G.; Bella, R.; Paulus, W.; Pennisi, M. Repetitive transcranial magnetic stimulation in stroke rehabilitation: Review of the current evidence and pitfalls. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419878317. [Google Scholar] [CrossRef] [Green Version]
- Teskey, G.C.; Flynn, C.; Goertzen, C.D.; Monfils, M.H.; Young, N.A. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol. Res. 2003, 25, 794–800. [Google Scholar] [CrossRef]
- Adkins, D.L.; Campos, P.; Quach, D.; Borromeo, M.; Schallert, K.; Jones, T.A. Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp. Neurol. 2006, 200, 356–370. [Google Scholar] [CrossRef]
- Adkins, D.L.; Hsu, J.E.; Jones, T.A. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp. Neurol. 2008, 212, 14–28. [Google Scholar] [CrossRef] [Green Version]
- Plautz, E.J.; Barbay, S.; Frost, S.B.; Friel, K.M.; Dancause, N.; Zoubina, E.V.; Stowe, A.M.; Quaney, B.M.; Nudo, R.J. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: A feasibility study in primates. Neurol. Res. 2003, 25, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Adkins-Muir, D.L.; Jones, T.A. Cortical electrical stimulation combined with rehabilitative training: Enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol. Res. 2003, 25, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Boychuk, J.A.; Adkins, D.L.; Kleim, J.A. Distributed versus focal cortical stimulation to enhance motor function and motor map plasticity in a rodent model of ischemia. Neurorehabil. Neural Repair 2011, 25, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Boychuk, J.A.; Schwerin, S.C.; Thomas, N.; Roger, A.; Silvera, G.; Liverpool, M.; Adkins, D.L.; Kleim, J.A. Enhanced motor recovery after stroke with combined cortical stimulation and rehabilitative training is dependent on infarct location. Neurorehabil. Neural Repair 2016, 30, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Bryant, A.J.; Adkins, D.L.; Sitko, A.A.; Combs, H.L.; Nordquist, S.K.; Jones, T.A. Enduring poststroke motor functional improvements by a well-timed combination of motor rehabilitative training and cortical stimulation in rats. Neurorehabil. Neural Repair 2016, 30, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Plautz, E.J.; Barbay, S.; Frost, S.B.; Zoubina, E.V.; Stowe, A.M.; Dancause, N.; Eisner-Janowicz, I.; Bury, S.D.; Taylor, M.D.; Nudo, R.J. Effects of subdural monopolar cortical stimulation paired with rehabilitative training on behavioral and neurophysiological recovery after cortical ischemic stroke in adult squirrel monkeys. Neurorehabil. Neural Repair 2016, 30, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Tennant, K.A.; Adkins, D.L.; Scalco, M.D.; Donlan, N.A.; Asay, A.L.; Thomas, N.; Kleim, J.A.; Jones, T.A. Skill learning induced plasticity of motor cortical representations is time and age-dependent. Neurobiol. Learn Mem. 2012, 98, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.A.; Lutsep, H.L.; Weinand, M.; Cramer, S.C. Motor cortex stimulation for the enhancement of recovery from stroke: A prospective, multicenter safety study. Neurosurgery 2006, 58, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Levy, R.; Ruland, S.; Weinand, M.; Lowry, D.; Dafer, R.; Bakay, R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: A multicenter feasibility study of safety and efficacy. J. Neurosurg. 2008, 108, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Plow, E.B.; Carey, J.R.; Nudo, R.J.; Pascual-Leone, A. Invasive cortical stimulation to promote recovery of function after stroke: A critical appraisal. Stroke 2009, 40, 1926–1931. [Google Scholar] [CrossRef]
- Levy, R.M.; Harvey, R.L.; Kissela, B.M.; Winstein, C.J.; Lutsep, H.L.; Parrish, T.B.; Cramer, S.C.; Venkatesan, L. Epidural electrical stimulation for stroke rehabilitation: Results of the prospective, multicenter, randomized, single-blinded everest trial. Neurorehabil. Neural Repair 2016, 30, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Lou, J.; Han, X.; Deng, Y.; Huang, X. Repetitive transcranial magnetic stimulation ameliorates cognitive impairment by enhancing neurogenesis and suppressing apoptosis in the hippocampus in rats with ischemic stroke. Front. Physiol. 2017, 8, 559. [Google Scholar] [CrossRef]
- Zhang, X.; Mei, Y.; Liu, C.; Yu, S. Effect of transcranial magnetic stimulation on the expression of c-Fos and brain-derived neurotrophic factor of the cerebral cortex in rats with cerebral infarct. J. Huazhong Univ. Sci. Technol. Med. Sci. 2007, 27, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Ge, H.; Zeng, H.; Yan, H.; Zhang, L.; Feng, H.; Chen, Y. Repetitive transcranial magnetic stimulation promotes neural stem cell proliferation and differentiation after intracerebral hemorrhage in mice. Cell Transpl. 2019, 28, 568–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, A.; Kim, J.H.; Pyo, S.; Jung, J.H.; Park, E.J.; Kim, S.H.; Cho, S.R. The differential effects of repetitive magnetic stimulation in an In Vitro neuronal model of ischemia/reperfusion injury. Front. Neurol. 2018, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Wang, S.; Guo, Y.; Wang, J.; Lou, M.; Wu, J.; Ding, M.; Tian, M.; Zhang, H. Protective effects of repetitive transcranial magnetic stimulation in a rat model of transient cerebral ischaemia: A microPET study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 954–961. [Google Scholar] [CrossRef]
- Guo, F.; Han, X.; Zhang, J.; Zhao, X.; Lou, J.; Chen, H.; Huang, X. Repetitive transcranial magnetic stimulation promotes neural stem cell proliferation via the regulation of MiR-25 in a rat model of focal cerebral ischemia. PLoS ONE 2014, 9, e109267. [Google Scholar] [CrossRef] [Green Version]
- Abbasnia, K.; Ghanbari, A.; Abedian, M.; Ghanbari, A.; Sharififar, S.; Azari, H. The effects of repetitive transcranial magnetic stimulation on proliferation and differentiation of neural stem cells. Anat. Cell Biol. 2015, 48, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Yoon, K.J.; Lee, Y.T.; Han, T.R. Mechanism of functional recovery after repetitive transcranial magnetic stimulation (rTMS) in the subacute cerebral ischemic rat model: Neural plasticity or anti-apoptosis? Exp. Brain Res. 2011, 214, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zheng, H.; Zhang, L.; Zhang, Q.; Li, L.; Pei, Z.; Hu, X. High-frequency repetitive transcranial magnetic stimulation (rTMS) improves functional recovery by enhancing neurogenesis and activating BDNF/TrkB signaling in ischemic rats. Int. J. Mol. Sci. 2017, 18, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Li, G.; Ma, C.; Chen, Y.; Wang, J.; Yang, Y. Repetitive magnetic stimulation promotes the proliferation of neural progenitor cells via modulating the expression of miR-106b. Int. J. Mol. Med. 2018, 42, 3631–3639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiki, M.; Kobayashi, H.; Abe, T.; Kamida, T. Repetitive transcranial magnetic stimulation for protection against delayed neuronal death induced by transient ischemia. J. Neurosurg. 2003, 99, 1063–1069. [Google Scholar] [CrossRef]
- Jackson, M.P.; Rahman, A.; Lafon, B.; Kronberg, G.; Ling, D.; Parra, L.C.; Bikson, M. Animal models of transcranial direct current stimulation: Methods and mechanisms. Clin. Neurophysiol. 2016, 127, 3425–3454. [Google Scholar] [CrossRef] [Green Version]
- Monai, H.; Ohkura, M.; Tanaka, M.; Oe, Y.; Konno, A.; Hirai, H.; Mikoshiba, K.; Itohara, S.; Nakai, J.; Iwai, Y.; et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat. Commun. 2016, 7, 11100. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef]
- Laakso, I.; Tanaka, S.; Koyama, S.; De Santis, V.; Hirata, A. Inter-subject variability in electric fields of motor cortical tDCS. Brain Stimul. 2015, 8, 906–913. [Google Scholar] [CrossRef]
- Notturno, F.; Pace, M.; Zappasodi, F.; Cam, E.; Bassetti, C.L.; Uncini, A. Neuroprotective effect of cathodal transcranial direct current stimulation in a rat stroke model. J. Neurol. Sci. 2014, 342, 146–151. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Cambiaghi, M.; Bacigaluppi, M.; Gallizioli, M.; Gaude, E.; Mari, S.; Sandrone, S.; Cursi, M.; Teneud, L.; Comi, G.; et al. Safety and efficacy of transcranial direct current stimulation in acute experimental ischemic stroke. Stroke 2013, 44, 3166–3174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.H.; Chan, S.J.; Pan, H.C.; Bandla, A.; King, N.K.K.; Wong, P.T.H.; Chen, Y.Y.; Ng, W.H.; Thakor, N.V.; Liao, L.D. Integrated treatment modality of cathodal-transcranial direct current stimulation with peripheral sensory stimulation affords neuroprotection in a rat stroke model. Neurophotonics 2017, 4, 045002. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Xu, R.X.; Zhang, A.W.; Di, W.; Xiao, Z.J.; Miao, J.Y.; Luo, N.; Fang, Y.N. Effects of transcranial direct current stimulation on hemichannel pannexin-1 and neural plasticity in rat model of cerebral infarction. Neuroscience 2012, 226, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.J.; Oh, B.M.; Kim, D.Y. Functional improvement and neuroplastic effects of anodal transcranial direct current stimulation (tDCS) delivered 1 day vs. 1 week after cerebral ischemia in rats. Brain Res. 2012, 1452, 61–72. [Google Scholar] [CrossRef]
- Braun, R.; Klein, R.; Walter, H.L.; Ohren, M.; Freudenmacher, L.; Getachew, K.; Ladwig, A.; Luelling, J.; Neumaier, B.; Endepols, H.; et al. Transcranial direct current stimulation accelerates recovery of function, induces neurogenesis and recruits oligodendrocyte precursors in a rat model of stroke. Exp. Neurol. 2016, 279, 127–136. [Google Scholar] [CrossRef]
- Liebetanz, D.; Koch, R.; Mayenfels, S.; Konig, F.; Paulus, W.; Nitsche, M.A. Safety limits of cathodal transcranial direct current stimulation in rats. Clin. Neurophysiol. 2009, 120, 1161–1167. [Google Scholar] [CrossRef]
- Jackson, M.P.; Truong, D.; Brownlow, M.L.; Wagner, J.A.; McKinley, R.A.; Bikson, M.; Jankord, R. Safety parameter considerations of anodal transcranial Direct Current Stimulation in rats. Brain Behav. Immun. 2017, 64, 152–161. [Google Scholar] [CrossRef]
- Morimoto, J.; Yasuhara, T.; Kameda, M.; Umakoshi, M.; Kin, I.; Kuwahara, K.; Kin, K.; Okazaki, M.; Takeuchi, H.; Sasaki, T.; et al. Electrical stimulation enhances migratory ability of transplanted bone marrow stromal cells in a rodent ischemic stroke model. Cell Physiol. Biochem. 2018, 46, 57–68. [Google Scholar] [CrossRef]
- Feng, J.F.; Liu, J.; Zhang, L.; Jiang, J.Y.; Russell, M.; Lyeth, B.G.; Nolta, J.A.; Zhao, M. Electrical guidance of human stem cells in the rat brain. Stem. Cell Reports 2017, 9, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Kremer, K.L.; Smith, A.E.; Sandeman, L.; Inglis, J.M.; Ridding, M.C.; Koblar, S.A. Transcranial magnetic stimulation of human adult stem cells in the mammalian brain. Front. Neural Circuits 2016, 10, 17. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.J.; Sha, R.; Li, M.X.; Chen, L.T.; Han, X.H.; Guo, F.; Chen, H.; Huang, X.L. Repetitive transcranial magnetic stimulation promotes functional recovery and differentiation of human neural stem cells in rats after ischemic stroke. Exp. Neurol. 2019, 313, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S.; Newton, J.M.; Swayne, O.B.; Lee, L.; Thompson, A.J.; Greenwood, R.J.; Rothwell, J.C.; Frackowiak, R.S. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 2006, 129, 809–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindenberg, R.; Renga, V.; Zhu, L.L.; Betzler, F.; Alsop, D.; Schlaug, G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 2010, 74, 280–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameli, M.; Grefkes, C.; Kemper, F.; Riegg, F.P.; Rehme, A.K.; Karbe, H.; Fink, G.R.; Nowak, D.A. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann. Neurol. 2009, 66, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.D.; Le, V.; Der-Yeghiaian, L.; See, J.; Newton, J.M.; Ward, N.S.; Cramer, S.C. Anatomy of stroke injury predicts gains from therapy. Stroke 2011, 42, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Nouri, S.; Cramer, S.C. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology 2011, 77, 1076–1083. [Google Scholar] [CrossRef] [Green Version]
- Wahl, A.S.; Omlor, W.; Rubio, J.C.; Chen, J.L.; Zheng, H.; Schroter, A.; Gullo, M.; Weinmann, O.; Kobayashi, K.; Helmchen, F.; et al. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science 2014, 344, 1250–1255. [Google Scholar] [CrossRef]
- Sommer, C.J.; Schabitz, W.R. Fostering poststroke recovery: Towards combination treatments. Stroke 2017, 48, 1112–1119. [Google Scholar] [CrossRef]
- Willett, N.J.; Boninger, M.L.; Miller, L.J.; Alvarez, L.; Aoyama, T.; Bedoni, M.; Brix, K.A.; Chisari, C.; Christ, G.; Dearth, C.L.; et al. Taking the next steps in regenerative rehabilitation: Establishment of a new interdisciplinary field. Arch. Phys. Med. Rehabil. 2020, 101, 917–923. [Google Scholar] [CrossRef]




| Reference | Model | Cell Therapy | Rehabilitation | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|
| Transplanted Cell | Cell Mass and Location | Timing of Transplantation | Category | Onset | Duration | Interactive Effect on Motor Function | Mechanism | ||
|
Hicks et al. [35] (2007) | MCAO in rats | Stem cells from mSVZ | 8 × 105 cells, ipsilateral sensorimotor cortex and striatum | 7 days after MCAO | Enriched environment | 8 days after MCAO | 30 days | n.a. | ↑Migration of transplanted cells |
| Hicks et al. [36] (2008) | Endothelin-1 induced MCAO in rats | Stem cells from mSVZ | 8 × 105 cells, ipsilateral sensorimotor cortex and striatum | 7 days after MCAO | Enriched environment | 8 days after MCAO | 3 months | n.a. | Majority (~99%) of cells died within 2 months of transplantation |
| Hicks et al. [37] (2009) | dMCAO in rats | hESC-derived NPCs | 8 × 105 cells, ipsilateral sensorimotor cortex | 7 days after dMCAO | Enriched environment | 8 days after MCAO | 66 days | No effect | Poor survival of transplanted cells |
| Seo et al. [38] (2013) | Hypoxic-ischemic brain injury in mice | hASCs | 1 × 105 cells, ipsilateral striatum | 5 weeks after injury | Enriched environment | 5 weeks after injury | 8 weeks | Synergistic | ↑Neurogenesis in striatum ↑Astrocytic activation |
| Zhang et al. [39] (2015) | MCAO in rats | rMSCs | 3 × 106 cells, intravenously | 1 day after MCAO | Treadmill exercise (4 m/min for the 1st day, 8 m/min for the 2nd day, 12 m/min for the remaining days for 20 min, every day) | 2 days after MCAO | 12 days | Synergistic | ↓Apoptosis |
| Cho et al. [40] (2016) | Hypoxic-ischemic brain injury in mice | hASCs | 1 × 105 cells, ipsilateral striatum | 5 weeks after injury | Enriched environment | 5 weeks after injury | 8 weeks | Synergistic | ↑Angiogenesis ↑Astrocytic activation |
| Sasaki et al. [41] (2016) | MCAO in rats | rMSCs | 1 × 106 cells, intravenously | 6 hours after MCAO | Treadmill exercise (3 m/min for 20 min, every day for 1 week. Speed was increased by 3 m/min every week) | 1 day after MCAO | 34 days | Synergistic | ↓Infarction volume ↑Corpus callosum thickness ↑Synaptogenesis |
| Zhao et al. [42] (2018) | MCAO in rats | rADSCs | 2 × 106 cells, intravenously | After common carotid artery reperfusion | Mild therapeutic hypothermia (33 °C) | During the ischemia | 2 hours | Additive or synergistic | ↓Infarction volume ↓Apoptosis ↑Angiogenesis ↓Glial scar formation ↓Inflammatory responses |
| Mu et al. [43] (2019) | MCAO in rats | hADMSCs | 2 × 106 cells, intravenously | 2 or 7 days after MCAO | Enriched environment | 2 days after MCAO | 42 days | Overlapping or additive | →Infarction volume →Angiogenesis →Glial scar formation |
| Reference | Model | Cell Therapy | Rehabilitation | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|
| Transplanted Cell | Cell Mass and Location | Timing of Transplantation | Category | Onset | Duration | Interactive Effect on Motor Function | Mechanism | ||
|
Kremer et al. [91] (2016) | Normal rats | Human dental pulp stem cells | 6 × 105 cells, right cortex and striatum | n.a. | Ipsilateral TMS (60% of the maximal output, 0.2 Hz for 15 min, every 2nd day, beginning on day 3 post-transplantation) | 2 days after transplantation | 12 days | Antagonistic | ↓Transplanted cell survival ↑Apoptosis |
| Morimoto et al. [89] (2018) | MCAO in rats | rMSCs | 2.5 × 105 cells, contralateral corpus callosum | 1 day after MCAO | Ipsilesional cathodal CS (100 µA, 100 Hz, continuously) | 1 day after MCAO | 14 days | n.a. | ↓Infarction volume ↑Transplanted cell migration ↑SDF-1α |
| Peng et al. [92] (2019) | MCAO in rats | hNSCs | 2.5 × 105 cells, ipsilateral striatum | 4 days after MCAO | Ipsilesional rTMS (26% of the maximal output, 10 Hz, 300 pulses/day, every day) | 5 days after MCAO | 28 days | Synergistic | ↑Neurogenesis in SVZ ↑BDNF/TrkB signaling pathway ↑Neural differentiation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, A.; Kubo, N.; Liang, N.; Aoyama, T.; Kuroki, H. Regenerative Rehabilitation for Stroke Recovery by Inducing Synergistic Effects of Cell Therapy and Neurorehabilitation on Motor Function: A Narrative Review of Pre-Clinical Studies. Int. J. Mol. Sci. 2020, 21, 3135. https://doi.org/10.3390/ijms21093135
Ito A, Kubo N, Liang N, Aoyama T, Kuroki H. Regenerative Rehabilitation for Stroke Recovery by Inducing Synergistic Effects of Cell Therapy and Neurorehabilitation on Motor Function: A Narrative Review of Pre-Clinical Studies. International Journal of Molecular Sciences. 2020; 21(9):3135. https://doi.org/10.3390/ijms21093135
Chicago/Turabian StyleIto, Akira, Naoko Kubo, Nan Liang, Tomoki Aoyama, and Hiroshi Kuroki. 2020. "Regenerative Rehabilitation for Stroke Recovery by Inducing Synergistic Effects of Cell Therapy and Neurorehabilitation on Motor Function: A Narrative Review of Pre-Clinical Studies" International Journal of Molecular Sciences 21, no. 9: 3135. https://doi.org/10.3390/ijms21093135
APA StyleIto, A., Kubo, N., Liang, N., Aoyama, T., & Kuroki, H. (2020). Regenerative Rehabilitation for Stroke Recovery by Inducing Synergistic Effects of Cell Therapy and Neurorehabilitation on Motor Function: A Narrative Review of Pre-Clinical Studies. International Journal of Molecular Sciences, 21(9), 3135. https://doi.org/10.3390/ijms21093135