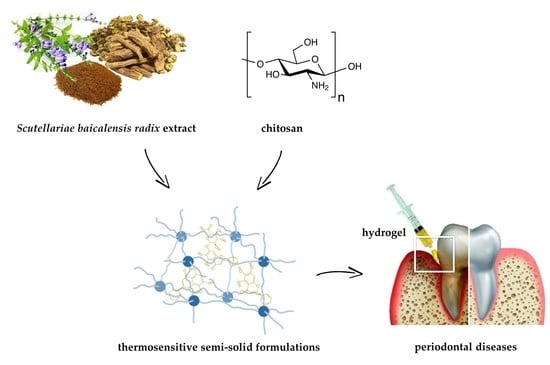
Development and Evaluation of Thermosensitive Hydrogels with Binary Mixture of Scutellariae baicalensis radix Extract and Chitosan for Periodontal Diseases Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of S. baicalensis radix Lyophilized Extract and Its Analysis
2.1.1. Extract Preparation
2.1.2. Determination of Flavonoids Content
2.2. Preformulation Studies of S. baicalensis radix Lyophilized Extract with Chitosan and the Evaluation of Their Activity
2.2.1. Binary Mixtures Preparation
2.2.2. ATR-FTIR Spectroscopy
2.2.3. Dissolution Studies
2.2.4. Permeability Studies
2.2.5. Biological Activity
2.3. Formulation Studies Involving S. baicalensis radix Lyophilized Extract with Chitosan and the Evaluation of Its Quality
2.3.1. Preparation of the Formulations and Rheological Analysis
2.3.2. In Vitro Dissolution Studies
2.3.3. Mucoadhesive Properties
3. Materials and Methods
3.1. Preparation of S. baicalensis radix Lyophilized Extract and Its Analysis
3.1.1. Extract Preparation
3.1.2. Determination of Flavonoids Content
3.2. Chemicals and Reagents
3.3. Preformulation Studies of S. baicalensis radix Lyophilized Extract with Chitosan and the Evaluation of Their Activity
3.3.1. Binary Mixtures Preparation
3.3.2. ATR-FTIR Spectroscopy
3.3.3. Dissolution Studies
3.3.4. Permeability Studies
3.3.5. Biological Activity
ABTS Assay
Ferrous Ion-Chelating Activity
Anti-Hyaluronidase Activity
Antimicrobial Activity
3.4. Formulation Studies Involving S. baicalensis radix Lyophilized Extract with Chitosan and the Evaluation of Its Quality
3.4.1. Hydrogels Preparation
3.4.2. Rheological Experiments
3.4.3. In Vitro Dissolution Studies
3.4.4. Mucoadhesive Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ara, T.; Nakatani, S.; Kobata, K.; Sogawa, N.; Sogawa, C. The Biological Efficacy of Natural Products against Acute and Chronic Inflammatory Diseases in the Oral Region. Medicines 2018, 5, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Trevisan, M.; Genco, R.J.; Dorn, J.P.; Falkner, K.L.; Sempos, C.T. Periodontal Disease and Risk of Cerebrovascular Disease: The First National Health and Nutrition Examination Survey and Its Follow-up Study. Arch. Intern. Med. 2000, 160, 2749–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, A.J.; Paskett, K.T.; Cheever, V.J.; Lipsky, M.S. Periodontitis: A Global Disease and the Primary Care Provider’s Role. Postgrad. Med. J. 2017, 93, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.M.; Reis, C.; Manzanares-Céspedes, M.C. Chronic Periodontitis, Inflammatory Cytokines, and Interrelationship with Other Chronic Diseases. Postgrad. Med. 2018, 130, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, C.; Dye, B.A. A Public Health Approach for Prevention of Periodontal Disease. Periodontology 2000 2020, 84, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Popova, C.; Dosseva-Panova, V.; Panov, V. Microbiology of Periodontal Diseases. A Review. Biotechnol. Biotechnol. Equip. 2013, 27, 3754–3759. [Google Scholar] [CrossRef]
- Akcali, A.; Huck, O.; Tenenbaum, H.; Davideau, J.L.; Buduneli, N. Periodontal Diseases and Stress: A Brief Review. J. Oral Rehabil. 2013, 40, 60–68. [Google Scholar] [CrossRef]
- Waddington, R.J.; Moseley, R.; Embery, G. Periodontal Disease Mechanisms: Reactive Oxygen Species: A Potential Role in the Pathogenesis of Periodontal Diseases. Oral Dis. 2000, 6, 138–151. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and Surgical Treatment of Periodontitis: How Many Options for One Disease? Periodontology 2000 2017, 75, 152–188. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Bianchi, S.; Tomei, A.R.; Continenza, M.A.; Macchiarelli, G. Microbiological and SEM-EDS Evaluation of Titanium Surfaces Exposed to Periodontal Gel: In Vitro Study. Materials 2019, 12, 1448. [Google Scholar] [CrossRef] [Green Version]
- Llor, C.; Bjerrum, L. Antimicrobial Resistance: Risk Associated with Antibiotic Overuse and Initiatives to Reduce the Problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, S.; Anderson, A.; Macchiarelli, G.; Hellwig, E.; Cieplik, F.; Vach, K.; Al-Ahmad, A. Subinhibitory Antibiotic Concentrations Enhance Biofilm Formation of Clinical Enterococcus Faecalis Isolates. Antibiotics 2021, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Puranik, M.; Harish, Y.; Anushri, M. Herbs: A Good Alternatives to Current Treatments for Oral Health Problems. Int. J. Adv. Health Sci. 2015, 1, 26–32. [Google Scholar]
- Eid Abdelmagyd, H.A.; Ram Shetty, D.S.; Musa Musleh Al-Ahmari, D.M. Herbal Medicine as Adjunct in Periodontal Therapies- A Review of Clinical Trials in Past Decade. J. Oral. Biol. Craniofac. Res. 2019, 9, 212–217. [Google Scholar] [CrossRef]
- Palombo, E.A. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evid.-Based Complementary Altern. Med. 2011, 2011, enep067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Chen, X.-Y.; Martin, C. Scutellaria Baicalensis, the Golden Herb from the Garden of Chinese Medicinal Plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Huang, Q.; Huang, B.; Lu, F. The Effect of Scutellaria Baicalensis Georgi on Immune Response in Mouse Model of Experimental Periodontitis. J. Dent. Sci. 2013, 8, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Lee, H.; Choi, Y.Y.; Lee, D.H.; Yang, W.M. Scutellaria Baicalensis Ameliorates the Destruction of Periodontal Ligament via Inhibition of Inflammatory Cytokine Expression. J. Chin. Med Assoc. 2018, 81, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, C.; Du, G.; Cao, Z. Protective Effects of Baicalin on Ligature-Induced Periodontitis in Rats. J. Periodontal Res. 2008, 43, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Z.-F.; Yang, L.-J. Influence of Baicalin on Alveolar Bone Resorption in Rat Experimental Periodontitis. Sci. Pharm. 2008, 76, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wu, Z.; Wan, L.; Wang, Q.; Chen, F. Influence of Baicalin on the Expression of Receptor Activator of Nuclear Factor-ΚB Ligand in Cultured Human Periodontal Ligament Cells. Pharmacology 2006, 77, 71–77. [Google Scholar] [CrossRef]
- Pei, Z.; Wang, B.; Zhang, F.; Niu, Z.; Shi, S.; Cannon, R.D.; Mei, L. Response of Human Periodontal Ligament Cells to Baicalin. J. Periodontol. 2014, 85, 1283–1290. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Kimura, A.; Tsuka, Y.; Horie, K.; Yoshimi, Y.; Awada, T.; Gunji, H.; Abe, T.; Nakajima, K.; Sakata, S.; et al. Baicalin Inhibits Root Resorption during Tooth Movement in a Rodent Model. Arch. Oral Biol. 2020, 116, 104770. [Google Scholar] [CrossRef]
- Li, C.; Cao, Z.; Yang, R.; Shang, Z.; Jin, L.; Cobert, E.F. Effects of baicalin on the expression of pro-MMP-1 and MMP-3 in human gingival fibroblasts and periodontal ligament cells. Zhonghua Kou Qiang Yi Xue Za Zhi 2004, 39, 197–200. [Google Scholar]
- Zhu, G.; Li, C.; Cao, Z. Inhibitory Effect of Flavonoid Baicalin on Degranulation of Human Polymorphonuclear Leukocytes Induced by Interleukin-8: Potential Role in Periodontal Diseases. J. Ethnopharmacol. 2007, 109, 325–330. [Google Scholar] [CrossRef]
- Ozma, M.A.; Khodadadi, E.; Pakdel, F.; Kamounah, F.S.; Yousefi, M.; Yousefi, B.; Asgharzadeh, M.; Ganbarov, K.; Kafil, H.S. Baicalin, a Natural Antimicrobial and Anti-Biofilm Agent. J. Herb. Med. 2021, 27, 100432. [Google Scholar] [CrossRef]
- Ren, M.; Zhao, Y.; He, Z.; Lin, J.; Xu, C.; Liu, F.; Hu, R.; Deng, H.; Wang, Y. Baicalein Inhibits Inflammatory Response and Promotes Osteogenic Activity in Periodontal Ligament Cells Challenged with Lipopolysaccharides. BMC Complementary Med. Ther. 2021, 21, 43. [Google Scholar] [CrossRef]
- Chen, L.-J.; Hu, B.-B.; Shi, X.-L.; Ren, M.-M.; Yu, W.-B.; Cen, S.-D.; Hu, R.-D.; Deng, H. Baicalein Enhances the Osteogenic Differentiation of Human Periodontal Ligament Cells by Activating the Wnt/β-Catenin Signaling Pathway. Arch. Oral Biol. 2017, 78, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; Bak, E.J.; Kim, M.; Kim, J.M.; Chung, W.-Y.; Cha, J.-H.; Yoo, Y.-J. Wogonin Inhibits Osteoclast Formation Induced by Lipopolysaccharide. Phytother. Res. 2010, 24, 964–968. [Google Scholar] [CrossRef]
- Arancibia, R.; Maturana, C.; Silva, D.; Tobar, N.; Tapia, C.; Salazar, J.C.; Martínez, J.; Smith, P.C. Effects of Chitosan Particles in Periodontal Pathogens and Gingival Fibroblasts. J. Dent Res. 2013, 92, 740–745. [Google Scholar] [CrossRef]
- Kmiec, M.; Pighinelli, L.; Tedesco, M.; Silva, M.; dos Reis, V. Chitosan-Properties and Applications in Dentistry. Adv. Tissue Eng. Regen. Med. 2017, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pignatello, R.; Basile, L.; Puglisi, G. Chitosan Glutamate Hydrogels with Local Anesthetic Activity for Buccal Application. Drug Deliv. 2009, 16, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Strandman, S.; Zhu, J.X.X.; Barralet, J.; Cerruti, M. Genipin-Crosslinked Catechol-Chitosan Mucoadhesive Hydrogels for Buccal Drug Delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Wang, B.; Booij-Vrieling, H.E.; Bronkhorst, E.M.; Shao, J.; Kouwer, P.H.J.; Jansen, J.A.; Walboomers, X.F.; Yang, F. Antimicrobial and Anti-Inflammatory Thermo-Reversible Hydrogel for Periodontal Delivery. Acta Biomater. 2020, 116, 259–267. [Google Scholar] [CrossRef]
- Ji, Q.X.; Zhao, Q.S.; Deng, J.; Lü, R. A Novel Injectable Chlorhexidine Thermosensitive Hydrogel for Periodontal Application: Preparation, Antibacterial Activity and Toxicity Evaluation. J. Mater. Sci. Mater. Med. 2010, 21, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Pakzad, Y.; Ganji, F. Thermosensitive Hydrogel for Periodontal Application: In Vitro Drug Release, Antibacterial Activity and Toxicity Evaluation. J. Biomater. Appl. 2016, 30, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling Properties of Purified Poloxamer 407. Heliyon 2017, 3. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Tasarek, S. Application of Gellan Gum in Pharmacy and Medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, S.; Bhattacharya, S.S.; Chattopadhyay, P. Trivalent Ion Cross-Linked PH Sensitive Alginate-Methyl Cellulose Blend Hydrogel Beads from Aqueous Template. Int. J. Biol. Macromol. 2013, 57, 297–307. [Google Scholar] [CrossRef]
- Wathoni, N.; Yuniarsih, N.; Cahyanto, A.; Muhctaridi, M. α-Mangostin Hydrogel Film Based Chitosan–Alginate for Recurrent Aphthous Stomatitis. Appl. Sci. 2019, 9, 5235. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.-F.; Hong, M.-H.; Ho, R.-M.; Chung, C.-K.; Lin, Y.-H.; Chen, C.-H.; Sung, H.-W. Novel Method Using a Temperature-Sensitive Polymer (Methylcellulose) to Thermally Gel Aqueous Alginate as a PH-Sensitive Hydrogel. Biomacromolecules 2004, 5, 1917–1925. [Google Scholar] [CrossRef]
- Bansal, K.; Rawat, M.K.; Jain, A.; Rajput, A.; Chaturvedi, T.P.; Singh, S. Development of Satranidazole Mucoadhesive Gel for the Treatment of Periodontitis. AAPS PharmSciTech 2009, 10, 716–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.C. Sol-Gel Behavior of Hydroxypropyl Methylcellulose (HPMC) in Ionic Media Including Drug Release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arweiler, N.B.; Pergola, G.; Kuenz, J.; Hellwig, E.; Sculean, A.; Auschill, T.M. Clinical and Antibacterial Effect of an Anti-Inflammatory Toothpaste Formulation with Scutellaria Baicalensis Extract on Experimental Gingivitis. Clin. Oral Investig. 2011, 15, 909–913. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Nam, S.-H. The Effects of Scutellariae Radix Extract Gargling Solution on the Prevention of Periodontal Disease. Biomed. Res. 2017, 0, 557–562. [Google Scholar]
- Patil, A.G.; Patil, P.; Jobanputra, A.H.; Verma, D. Herbal Formulations for Treatment of Dental Diseases: Perspectives, Potential, and Applications. In Engineering Interventions in Foods and Plants; Apple Academic Press: Florida, FA, USA, 2017. [Google Scholar]
- Pappu, R.; Varghese, J.; Koteshwara, K.; Kamath, V.; Lobo, R.; Nimmy, K. Evaluation of Biodegradable Gel Containing Flax Seed Extract (Linum Usitatissimum) as a Targeted Drug Delivery for Management of Chronic Periodontitis. J. Herb. Med. 2019, 15, 100254. [Google Scholar] [CrossRef]
- Pai, M.R.; Acharya, L.D.; Udupa, N. Evaluation of Antiplaque Activity of Azadirachta Indica Leaf Extract Gel—a 6-Week Clinical Study. J. Ethnopharmacol. 2004, 90, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Safiaghdam, H.; Oveissi, V.; Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal Plants for Gingivitis: A Review of Clinical Trials. Iran J. Basic. Med. Sci. 2018, 21, 978–991. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Forouzanfar, A.; Sathyapalan, T.; Orafai, H.M.; Sahebkar, A. Curcumin for the Management of Periodontal Diseases: A Review. Curr. Pharm. Des. 2020, 26, 4277–4284. [Google Scholar] [CrossRef]
- Prabaharan, M.; Mano, J.F. Chitosan-Based Particles as Controlled Drug Delivery Systems. Drug Deliv. 2004, 12, 41–57. [Google Scholar] [CrossRef]
- European Medicines Agency. European Pharmacopoeia (04/2011:2438) Monograph Scutellariae Baicalensis Radix; EDQM Council of Europe: Strasbourg, France, 2011; pp. 1262–1263. [Google Scholar]
- Lee, K.J.; Jung, P.M.; Oh, Y.-C.; Song, N.-Y.; Kim, T.; Ma, J.Y. Extraction and Bioactivity Analysis of Major Flavones Compounds from Scutellaria Baicalensis Using In Vitro Assay and Online Screening HPLC-ABTS System. Available online: https://www.hindawi.com/journals/jamc/2014/563702/ (accessed on 19 February 2021).
- Wang, R.; Luo, J.; Kong, L. Screening of Radical Scavengers in Scutellaria Baicalensis Using HPLC with Diode Array and Chemiluminescence Detection. J. Sep. Sci. 2012, 35, 2223–2227. [Google Scholar] [CrossRef]
- ICH. Validation of analytical procedures Q2(R2). In Proceedings of the International Conference of Harmonisation, Geneva, Switzerland, 14 November 2018. [Google Scholar]
- Unsalan, O.; Erdogdu, Y.; Gulluoglu, M.T. FT-Raman and FT-IR Spectral and Quantum Chemical Studies on Some Flavonoid Derivatives: Baicalein and Naringenin. J. Raman Spectrosc. 2009, 40, 562–570. [Google Scholar] [CrossRef]
- Xu, C.-H.; Liu, S.-L.; Zhao, S.-N.; Li, S.-Z.; Sun, S.-Q. Unveiling Ontogenesis of Herbal Medicine in Plant Chemical Profiles by Infrared Macro-Fingerprinting. Planta Med. 2013, 79, 1068–1076. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Cheng, Y.; Wang, Z.; Huang, D.; Miao, H.; Zhang, Y. Preparation and Characterization of Baicalein Powder Micronized by the SEDS Process. J. Supercrit. Fluids 2015, 104, 177–182. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Grimling, B.; Jasińska, J.; Meler, J.; Szcześniak, M.; Pluta, J.; Górniak, A. Physicochemical Characterization and Dissolution Studies of Solid Dispersions of Clotrimazole with Chitosan. Prog. Chem. Appl. Chitin Its Deriv. 2016, 21. [Google Scholar] [CrossRef]
- Wang, X.; Tang, R.; Zhang, Y.; Yu, Z.; Qi, C. Preparation of a Novel Chitosan Based Biopolymer Dye and Application in Wood Dyeing. Polymers 2016, 8, 338. [Google Scholar] [CrossRef]
- Oh, J.-W.; Chun, S.C.; Chandrasekaran, M. Preparation and In Vitro Characterization of Chitosan Nanoparticles and Their Broad-Spectrum Antifungal Action Compared to Antibacterial Activities against Phytopathogens of Tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Maestrelli, F.; Zerrouk, N.; Chemtob, C.; Mura, P. Influence of Chitosan and Its Glutamate and Hydrochloride Salts on Naproxen Dissolution Rate and Permeation across Caco-2 Cells. Int. J. Pharm. 2004, 271, 257–267. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Mernea, M.; Buiu, C.; Avram, S. Scutellaria Baicalensis Flavones as Potent Drugs against Acute Respiratory Injury during SARS-CoV-2 Infection: Structural Biology Approaches. Processes 2020, 8, 1468. [Google Scholar] [CrossRef]
- Song, J.-W.; Long, J.-Y.; Xie, L.; Zhang, L.-L.; Xie, Q.-X.; Chen, H.-J.; Deng, M.; Li, X.-F. Applications, Phytochemistry, Pharmacological Effects, Pharmacokinetics, Toxicity of Scutellaria Baicalensis Georgi. and Its Probably Potential Therapeutic Effects on COVID-19: A Review. Chin. Med. 2020, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Sohi, H.; Ahuja, A.; Ahmad, F.J.; Khar, R.K. Critical Evaluation of Permeation Enhancers for Oral Mucosal Drug Delivery. Drug Dev. Ind. Pharm. 2010, 36, 254–282. [Google Scholar] [CrossRef]
- Liang, W.; Huang, X.; Chen, W. The Effects of Baicalin and Baicalein on Cerebral Ischemia: A Review. Aging Dis. 2017, 8, 850–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akao, T.; Kawabata, K.; Yanagisawa, E.; Ishihara, K.; Mizuhara, Y.; Wakui, Y.; Sakashita, Y.; Kobashi, K. Baicalin, the Predominant Flavone Glucuronide of Scutellariae Radix, Is Absorbed from the Rat Gastrointestinal Tract as the Aglycone and Restored to Its Original Form. J. Pharm. Pharmacol. 2000, 52, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Varela-López, A.; Bullón, P.; Giampieri, F.; Quiles, J.L. Non-Nutrient, Naturally Occurring Phenolic Compounds with Antioxidant Activity for the Prevention and Treatment of Periodontal Diseases. Antioxidants 2015, 4, 447–481. [Google Scholar] [CrossRef]
- Ramesh, A.; Varghese, S.S.; Doraiswamy, J.N.; Malaiappan, S. Herbs as an Antioxidant Arsenal for Periodontal Diseases. J. Intercult. Ethnopharmacol. 2016, 5, 92–96. [Google Scholar] [CrossRef]
- Talmaç, A.C.; Çalişir, M. Antioxidants and Periodontal Diseases. Gingival Dis.-A Prof. Approach Treat. Prev. 2019. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Amorati, R.; Valgimigli, L. Methods to Measure the Antioxidant Activity of Phytochemicals and Plant Extracts. J. Agric. Food Chem. 2018, 66, 3324–3329. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Nallamuthu, I.; Devi, A.; Khanum, F. Chlorogenic Acid Loaded Chitosan Nanoparticles with Sustained Release Property, Retained Antioxidant Activity and Enhanced Bioavailability. Asian J. Pharm. Sci. 2015, 10, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Fan, H.; Yin, P.; Yang, L.; Xue, Q.; Li, X.; Sun, L.; Liu, Y. Structure-Activity Relationship of Eight High Content Flavonoids Analyzed with a Preliminary Assign-Score Method and Their Contribution to Antioxidant Ability of Flavonoids-Rich Extract from Scutellaria Baicalensis Shoots. Arab. J. Chem. 2018, 11, 159–170. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Fang, Z.; Hu, Y.; Chen, S.; Sugawara, T.; Ye, X. The Effect of the Molecular Architecture on the Antioxidant Properties of Chitosan Gallate. Mar. Drugs 2016, 14, 95. [Google Scholar] [CrossRef] [Green Version]
- Kamil, J.Y.V.A.; Jeon, Y.-J.; Shahidi, F. Antioxidative Activity of Chitosans of Different Viscosity in Cooked Comminuted Flesh of Herring (Clupea Harengus). Food Chem. 2002, 79, 69–77. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant Activity of Water-Soluble Chitosan Derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Perez, C.A.; Wei, Y.; Guo, M. Iron-Binding and Anti-Fenton Properties of Baicalein and Baicalin. J. Inorg. Biochem. 2009, 103, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In Vitro Analysis of Iron Chelating Activity of Flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Yamalik, N.; Kilinç, K.; Caglayan, F.; Eratalay, K.; Caglayan, G. Molecular Size Distribution Analysis of Human Gingival Proteoglycans and Glycosaminoglycans in Specific Periodontal Diseases. J. Clin. Periodontol. 1998, 25, 145–152. [Google Scholar] [CrossRef]
- Scheibner, K.A.; Lutz, M.A.; Boodoo, S.; Fenton, M.J.; Powell, J.D.; Horton, M.R. Hyaluronan Fragments Act as an Endogenous Danger Signal by Engaging TLR2. J. Immunol. 2006, 177, 1272–1281. [Google Scholar] [CrossRef] [Green Version]
- Tanimoto, K.; Nakatani, Y.; Tanaka, N.; Ueki, M.; Yanagida, T.; Kitamura, R.; Tanne, Y.; Lin, Y.Y.; Kunimatsu, R.; Tanne, K. Inhibition of the Proliferation of Human Periodontal Ligament Fibroblasts by Hyaluronidase. Arch. Oral Biol. 2008, 53, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, P.; Kamal, R. Hyaluronic Acid: A Boon in Periodontal Therapy. N. Am. J. Med. Sci. 2013, 5, 309–315. [Google Scholar] [CrossRef]
- Moseley, R.; Waddington, R.J.; Embery, G. Hyaluronan and Its Potential Role in Periodontal Healing. Dent Update 2002, 29, 144–148. [Google Scholar] [CrossRef]
- Powell, J.D.; Horton, M.R. Threat Matrix. Low-molecular-weight hyaluronan (HA) as a danger signal. Immunol. Res. 2005, 31, 207–218. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a Crucial Regulator of Inflammation. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Kuppusamy, U.R.; Khoo, H.E.; Das, N.P. Structure-Activity Studies of Flavonoids as Inhibitors of Hyaluronidase. Biochem. Pharmacol. 1990, 40, 397–401. [Google Scholar] [CrossRef]
- Nema, N.K.; Maity, N.; Sarkar, B.K.; Mukherjee, P.K. Matrix Metalloproteinase, Hyaluronidase and Elastase Inhibitory Potential of Standardized Extract of Centella Asiatica. Pharm. Biol. 2013, 51, 1182–1187. [Google Scholar] [CrossRef]
- Liana, L.; Rizal, R.; Widowati, W.; Lee, F.; Akbar, K.; Fachrial, E.; Lister, I.N. Antioxidant and Anti-Hyaluronidase Activities of Dragon Fruit Peel Extract and Kaempferol-3-O-Rutinoside. J. Kedokt. Brawijaya 2019, 30, 247. [Google Scholar] [CrossRef]
- Kakegawa, H.; Matsumoto, H.; Satoh, T. Inhibitory Effects of Some Natural Products on the Activation of Hyaluronidase and Their Anti-Allergic Actions. Chem. Pharm. Bull. 1992, 40, 1439–1442. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Kim, G.-H. Evaluation of Antioxidant and Inhibitory Activities for Different Subclasses Flavonoids on Enzymes for Rheumatoid Arthritis. J. Food Sci. 2010, 75, H212–H217. [Google Scholar] [CrossRef]
- Zeng, H.; Hu, G.; You, J.; Yang, R.; Qu, L. Spectroscopic and Molecular Modeling Investigation on the Interactions between Hyaluronidase and Baicalein and Chrysin. Process. Biochem. 2015, 50, 738–745. [Google Scholar] [CrossRef]
- Tigga, K.; Marandi, U.; Samdershi, D.; Besra, S. In-Vitro Hyaluronidase Inhibition Assay of Chitosan Extracted from Exoskeleton of Freshwater Edible Crab Sartoriana Spinigera. J. Exp. Zool. India 2019, 22, 1229–1233. [Google Scholar]
- Mao, S.; Liu, X.; Xia, W. Chitosan Oligosaccharide-g-Linalool Polymer as Inhibitor of Hyaluronidase and Collagenase Activity. Int. J. Biol. Macromol. 2021, 166, 1570–1577. [Google Scholar] [CrossRef]
- Carrol, D.H.; Chassagne, F.; Dettweiler, M.; Quave, C.L. Antibacterial Activity of Plant Species Used for Oral Health against Porphyromonas Gingivalis. PLoS ONE 2020, 15, e0239316. [Google Scholar] [CrossRef]
- Da, X.; Nishiyama, Y.; Tie, D.; Hein, K.Z.; Yamamoto, O.; Morita, E. Antifungal Activity and Mechanism of Action of Ou-Gon (Scutellaria Root Extract) Components against Pathogenic Fungi. Sci. Rep. 2019, 9, 1683. [Google Scholar] [CrossRef]
- Yin, B.; Li, W.; Qin, H.; Yun, J.; Sun, X. The Use of Chinese Skullcap (Scutellaria Baicalensis) and Its Extracts for Sustainable Animal Production. Animals 2021, 11, 1039. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D. de Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Fahmy, M.M. Synthesis and Antimicrobial Activity of Some Novel Cross-Linked Chitosan Hydrogels. Int. J. Mol. Sci. 2012, 13, 11194–11209. [Google Scholar] [CrossRef]
- Yaprak (Hızarcıoğlu) Karavana, S.; Güneri, P.; Ertan, G. Benzydamine Hydrochloride Buccal Bioadhesive Gels Designed for Oral Ulcers: Preparation, Rheological, Textural, Mucoadhesive and Release Properties. Pharm. Dev. Technol. 2009, 14, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Guneri, T. Rheological and Mechanical Properties of Poloxamer Mixtures as a Mucoadhesive Gel Base. Pharm. Dev. Technol. 2011, 16, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, V.M. On the Applicability of the Ostwald–De Waele Model in Solving Applied Problems. J. Eng. Phys. Thermophy. 2017, 90, 1213–1218. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Song, J.-Y.; Lee, E.-J.; Park, S.-K. Rheological Properties and Microstructures of Carbopol Gel Network System. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Lopez, C.G.; Richtering, W. Oscillatory Rheology of Carboxymethyl Cellulose Gels: Influence of Concentration and PH. Carbohydr. Polym. 2021, 267, 118117. [Google Scholar] [CrossRef]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Importance of Viscoelastic Property Measurement of a New Hydrogel for Health Care. AIP Conf. Proc. 2009, 1152, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Novel Hydrogels of PVP–CMC and Their Swelling Effect on Viscoelastic Properties. J. Appl. Polym. Sci. 2010, 117, 1703–1710. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.-F.; Rouse, J.J.; Sanderson, F.D.; Meidan, V.; Eccleston, G.M. Validation of a Static Franz Diffusion Cell System for In Vitro Permeation Studies. AAPS PharmSciTech 2010, 11, 1432–1441. [Google Scholar] [CrossRef] [Green Version]
- Zillich, O.V.; Schweiggert-Weisz, U.; Hasenkopf, K.; Eisner, P.; Kerscher, M. Release and in vitro skin permeation of polyphenols from cosmetic emulsions. Int. J. Cosmet. Sci. 2013, 35, 491–501. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Serpe, L.; Martinelli, C.C.M.; da Silva, C.B.; Santos, C.P.D.; Novaes, P.D.; Volpato, M.C.; de Paula, E.; Lopez, R.F.V.; Groppo, F.C. Evaluation of Different Pig Oral Mucosa Sites as Permeability Barrier Models for Drug Permeation Studies. Eur. J. Pharm. Sci. 2016, 81, 52–59. [Google Scholar] [CrossRef]
- Swarbrick, J. Encyclopedia of Pharmaceutical Technology, 3rd ed.; Tom 2; Informa Healthcare: New York, NY, USA, 2007. [Google Scholar]
- Dissolution test for solid dosage forms. In European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020; Chapter 2.9.3.
- Moore, J.W.; Flanner, H.H. Mathematical Comparison of Dissolution Profiles. Pharm. Technol. 1996, 20, 64–75. [Google Scholar]
- Paczkowska, M.; Chanaj-Kaczmarek, J.; Romaniuk-Drapała, A.; Rubiś, B.; Szymanowska, D.; Kobus-Cisowska, J.; Szymańska, E.; Winnicka, K.; Cielecka-Piontek, J. Mucoadhesive Chitosan Delivery System with Chelidonii Herba Lyophilized Extract as a Promising Strategy for Vaginitis Treatment. J. Clin. Med. 2020, 9, 1208. [Google Scholar] [CrossRef] [Green Version]
- Fischer, H.; Kansy, M.; Avdeef, A.; Senner, F. Permeation of Permanently Positive Charged Molecules through Artificial Membranes--Influence of Physico-Chemical Properties. Eur. J. Pharm. Sci. 2007, 31, 32–42. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Dudek-Makuch, M.; Chanaj-Kaczmarek, J.; Czepulis, N.; Korybalska, K.; Rutkowski, R.; Łuczak, J.; Grabowska, K.; Bylka, W.; Witowski, J. Anti-Inflammatory Activity and Phytochemical Profile of Galinsoga Parviflora Cav. Molecules 2018, 23, 2133. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Szymaǹska, E.; Sosnowska, K.; Miltyk, W.; Rusak, M.; Basa, A.; Winnicka, K. The Effect of β-Glycerophosphate Crosslinking on Chitosan Cytotoxicity and Properties of Hydrogels for Vaginal Application. Polymers 2015, 7, 2223–2244. [Google Scholar] [CrossRef] [Green Version]
- Paczkowska-Walendowska, M.; Dvořák, J.; Rosiak, N.; Tykarska, E.; Szymańska, E.; Winnicka, K.; Ruchała, M.A.; Cielecka-Piontek, J. Buccal Resveratrol Delivery System as a Potential New Concept for the Periodontitis Treatment. Pharmaceutics 2021, 13, 417. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; Ferreira, S.B.D.S.; Reis, A.V.; Cook, M.T.; Bruschi, M.L. Assessing Mucoadhesion in Polymer Gels: The Effect of Method Type and Instrument Variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef] [Green Version]







| Papp(× 10−6 cm s−1) | |||
|---|---|---|---|
| Baicalin | Baicalein | Wogonin | |
| Standard | 0.02 ± 0.01 | 91.56 ± 2.72 | 57.23 ± 5.42 |
| SBE | n.d. | 116.58 ± 3.97 | 134.43 ± 3.59 |
| SBE/Cs 80:500 | n.d. | 119.05 ± 7.64 | 133.66 ± 6.44 |
| SBE/Cs 80:1000 | n.d. | 129.03 ± 1.34 | 130.60 ± 3.58 |
| Pathogen | MIC (µg mL−1) | ||||||
|---|---|---|---|---|---|---|---|
| SBE | SBE/Cs 80:500 | SBE/Cs 80:1000 | Cs 80:500 | Cs 80:1000 | Baicalin | Baicalein | |
| A. naeslundii | 1250 | >2500 | >2500 | 1250 | 1250 | 312.5 | 156.25 |
| L. acidophilus | 625 | 39.1 * | >2500 | 78.1 | 156.25 | 156.25 | 312.5 |
| S. aureus | 1250 | 78.125 * | 2500 | 156.25 | 2500 | 625 | 625 |
| S. epidermidis | 1250 | 78.125 * | >2500 | 156.25 | >2500 | 625 | 625 |
| S. mutans Clarke ATCC 25175 | >2500 | 156.25 * | >2500 | 78.1 | 156.25 | 625 | 312.5 |
| E. coli | 1250 | 312.5 * | 2500 | 625 | 2500 | 1250 | 312.5 |
| P. mirabilis | 1250 | 2500 | 2500 | 2500 | 2500 | 1250 | 1250 |
| P. intermedia ATCC 25611 | 625 | 1250 | >2500 | 1250 | 1250 | 1250 | 625 |
| C. albicans | 1250 | 156.25 * | 2500 | 312.5 | 2500 | 625 | 1250 |
| C. tropicalis | 1250 | 156.25 * | 2500 | 312.5 | 2500 | 1250 | 625 |
| P1 | P2 | F1-2 | F1-4 | F2-2 | F2-4 | |
|---|---|---|---|---|---|---|
| Controlled rate | ||||||
| K | 188.53 ± 5.42 | 9.07 ± 0.64 | 186.67 ± 4.00 | 113.17 ± 7.06 | --- | --- |
| n | 0.11 ± 0.00 | 0.74 ± 0.02 | 0.10 ± 0.00 | 0.11 ± 0.00 | --- | --- |
| τ0 | --- | 316.53 ± 8.46 | --- | --- | --- | --- |
| Temperature sweeping | ||||||
| Tsol–gel | 26.65 ± 0.11 | 25.68 ± 0.02 | 26.75 ± 0.04 | 26.28 ± 0.03 | 26.62 ± 0.02 | 25.80 ± 0.02 |
| ηsol–gel | 8.45 ± 0.07 | 13.02 ± 0.47 | 12.21 ± 0.04 | 9.62 ± 0.03 | 10.61 ± 0.05 | 16.93 ± 0.03 |
| Stress sweeping | ||||||
| LVR | 74.83 ± 9.05 | 106.34 ± 10.01 | 84.48 ± 20.90 | 66.31 ± 18.08 | 62.07 ± 10.13 | 70.63 ± 13.13 |
| Cross-over ↑ | 158.43 ± 1.40 | 189.87 ± 5.09 | 165.97 ± 13.90 | 147.82 ± 20.64 | 168.77 ± 11.12 | 195.77 ± 10.37 |
| Cross-over ↓ | 92.88 ± 3.13 | 122.67 ± 12.19 | 92.73 ± 0.35 | 89.10 ± 5.15 | 87.73 ± 4.10 | 122.10 ± 7.24 |
| Component | AlgNa | MC | HPC | PEG400 | P407 | DW | SBE/Cs 80:500 |
|---|---|---|---|---|---|---|---|
| P1 | 0.4 | 0.6 | --- | 2.0 | 17.0 | 80.0 | --- |
| P2 | 0.4 | --- | 0.6 | 2.0 | 17.0 | 80.0 | --- |
| F1-2 | P1 98.0 | 2.0 | |||||
| F1-4 | P1 96.0 | 4.0 | |||||
| F2-2 | P2 98.0 | 2.0 | |||||
| F2-4 | P2 96.0 | 4.0 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanaj-Kaczmarek, J.; Osmałek, T.; Szymańska, E.; Winnicka, K.; Karpiński, T.M.; Dyba, M.; Bekalarska-Dębek, M.; Cielecka-Piontek, J. Development and Evaluation of Thermosensitive Hydrogels with Binary Mixture of Scutellariae baicalensis radix Extract and Chitosan for Periodontal Diseases Treatment. Int. J. Mol. Sci. 2021, 22, 11319. https://doi.org/10.3390/ijms222111319
Chanaj-Kaczmarek J, Osmałek T, Szymańska E, Winnicka K, Karpiński TM, Dyba M, Bekalarska-Dębek M, Cielecka-Piontek J. Development and Evaluation of Thermosensitive Hydrogels with Binary Mixture of Scutellariae baicalensis radix Extract and Chitosan for Periodontal Diseases Treatment. International Journal of Molecular Sciences. 2021; 22(21):11319. https://doi.org/10.3390/ijms222111319
Chicago/Turabian StyleChanaj-Kaczmarek, Justyna, Tomasz Osmałek, Emilia Szymańska, Katarzyna Winnicka, Tomasz M. Karpiński, Magdalena Dyba, Marta Bekalarska-Dębek, and Judyta Cielecka-Piontek. 2021. "Development and Evaluation of Thermosensitive Hydrogels with Binary Mixture of Scutellariae baicalensis radix Extract and Chitosan for Periodontal Diseases Treatment" International Journal of Molecular Sciences 22, no. 21: 11319. https://doi.org/10.3390/ijms222111319
APA StyleChanaj-Kaczmarek, J., Osmałek, T., Szymańska, E., Winnicka, K., Karpiński, T. M., Dyba, M., Bekalarska-Dębek, M., & Cielecka-Piontek, J. (2021). Development and Evaluation of Thermosensitive Hydrogels with Binary Mixture of Scutellariae baicalensis radix Extract and Chitosan for Periodontal Diseases Treatment. International Journal of Molecular Sciences, 22(21), 11319. https://doi.org/10.3390/ijms222111319