Increase in the Antioxidant and Anti-Inflammatory Activity of Euterpe oleracea Martius Oil Complexed in β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Activity of EOO, βCD, HPβCD, EOO-βCD, and EOO-HPβCD
2.1.1. Hydroxyl Radical (OH·) Scavenging Activity
2.1.2. Total Antioxidant Capacity (TAC)
2.1.3. Reducing Power (RP)
2.2. Anti-Inflammatory Activity of EOO and EOO-βCD Complex
2.2.1. Effect of EOO and EOO-βCD in a Model of Paw Edema Induced by Carrageenan in Mice
2.2.2. Effect of EOO and EOO-βCD in a Model of Air Pouch Induced by Carrageenan in Mice
3. Discussion
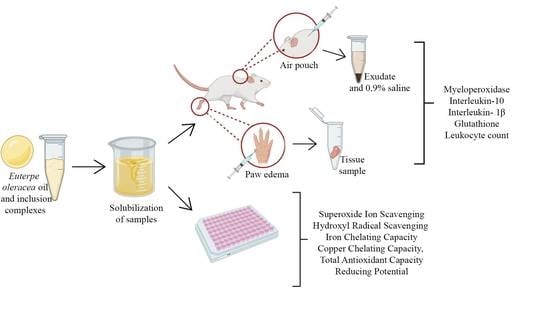
4. Materials and Methods
4.1. Materials
4.2. In Vitro Antioxidant Activity of EOO, EOO-βCD and EOO-HPβCD
4.3. In Vivo Anti-Inflammatory Activity of EOO-βCD
4.3.1. Animals
4.3.2. Carrageenan-Induced Paw Edema Test
4.3.3. Carrageenan Air Pouch
4.3.4. Myeloperoxidase Activity (MPO)
4.3.5. Cytokine Dosage—Interleukins 1-β (IL-1β) and 10 (IL-10)
4.3.6. Glutathione (GSH)
4.3.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 6th ed.; W.H. Freeman: New York, NY, USA, 2013; ISBN 9781464109621. [Google Scholar]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S.; Baker, A.; Baker, D.L. Cellular and Molecular Immunology; Elsevier Saunders: Philadelphia, PA, USA, 2015; ISBN 9780323222754. [Google Scholar]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, C.X.; Gustafson, B.; Hammarstedt, A.; Hedjazifar, S.; Smith, U. Inflamed adipose tissue, insulin resistance and vascular injury. Diabetes Metab. Res. Rev. 2008, 24, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, B.Y.; Ithurburn, M.P.; Rigsbee, C.A.; Bridges, S.L.; Moellering, D.R.; Gower, B.; Bamman, M. Chronic inflammation in rheumatoid arthritis and mediators of skeletal muscle pathology and physical impairment: A review. Arthritis Care Res. 2019, 71, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Ptaschinski, C.; Lukacs, N.W. Acute and chronic inflammation induces disease pathogenesis. Mol. Pathol. 2018, 1, 25–43. [Google Scholar] [CrossRef]
- De Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; Converti, A.; Neves de Lima, Á.A. The use of Euterpe oleracea Mart. as a new perspective for disease treatment and prevention. Biomolecules 2020, 10, 813. [Google Scholar] [CrossRef]
- Macedo, M. Contribuição ao Estudo de Plantas Econômicas no Estado de Mato Grosso; Edufmt: Cuiabá, Brazil, 1995; ISBN 9788532700391. [Google Scholar]
- De Yamaguchi, K.K.L.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Talcott, S.T.; Safe, S.; Mertens-Talcott, S. Absorption and biological activity of phytochemical-rich extracts from açai (Euterpe oleracea Mart.) pulp and oil in vitro. J. Agric. Food Chem. 2008, 56, 3593–3600. [Google Scholar] [CrossRef]
- Agawa, S.; Sakakibara, H.; Iwata, R.; Shimoi, K.; Hergesheimer, A.; Kumazawa, S. Anthocyanins in mesocarp/epicarp and endocarp of fresh acai (Euterpe oleracea Mart.) and their antioxidant activities and bioavailability. Food Sci. Technol. Res. 2011, 17, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain bv-2 microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.-E.; Vincken, J.-P.; Gruppen, H. Polyphenolic composition and antioxidant activity of açai (Euterpe oleracea Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Cedrim, P.C.A.S.; Barros, E.M.A.; do Nascimento, T.G. Antioxidant properties of acai (Euterpe oleracea) in the metabolic syndrome. Braz. J. Food Technol. 2018, 21, 2017092. [Google Scholar] [CrossRef]
- De Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; Kawashima Pacheco, S.Y.; da Silva, S.S.; Barbosa, E.G.; Pereira, R.R.; Costa, R.M.R.; Silva Junior, J.O.C.; da Silva Ferreira, M.A.; de Almeida, J.C.; et al. Development and evaluation of antimicrobial and modulatory activity of inclusion complex of Euterpe oleracea Mart oil and β-cyclodextrin or HP-β-cyclodextrin. Int. J. Mol. Sci. 2020, 21, 942. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, Y.M.B.G.; Menezes, P.P.; Sousa, B.M.H.; Lima, B.S.; Trindade, I.A.S.; Serafini, M.R.; Pereira, E.W.M.; Rezende, M.M.; Quintans, J.S.S.; Quintans-Júnior, L.J.; et al. Inclusion complex between β-cyclodextrin and hecogenin acetate produces superior analgesic effect in animal models for orofacial pain. Biomed. Pharmacother. 2017, 93, 754–762. [Google Scholar] [CrossRef]
- Costa, M.D.S.; Rocha, J.E.; Campina, F.F.; Silva, A.R.P.; Da Cruz, R.P.; Pereira, R.L.S.; Quintans-Júnior, L.J.; De Menezes, I.R.A.; Adriano, A.D.S.; De Freitas, T.S.; et al. Comparative analysis of the antibacterial and drug-modulatory effect of D-limonene alone and complexed with β-cyclodextrin. Eur. J. Pharm. Sci. 2019, 128, 158–161. [Google Scholar] [CrossRef]
- De Oliveira, L.C.; de Menezes, D.L.B.; da Silva, V.C.; Lourenço, E.M.G.; Miranda, P.H.S.; de Silva, M.D.J.A.; Lima, E.S.; da Júnior, V.F.V.; Marreto, R.N.; Converti, A.; et al. In silico study, physicochemical, and in vitro lipase inhibitory activity of α,β-amyrenone inclusion complexes with cyclodextrins. Int. J. Mol. Sci. 2021, 22, 9882. [Google Scholar] [CrossRef]
- Andrade, T.A.; Freitas, T.S.; Araújo, F.O.; Menezes, P.P.; Dória, G.A.A.; Rabelo, A.S.; Quintans-Júnior, L.J.; Santos, M.R.V.; Bezerra, D.P.; Serafini, M.R.; et al. Physico-chemical characterization and antibacterial activity of inclusion complexes of Hyptis martiusii Benth essential oil in β-cyclodextrin. Biomed. Pharmacother. 2017, 89, 201–207. [Google Scholar] [CrossRef]
- Silva, J.C.; Almeida, J.R.G.S.; Quintans, J.S.S.; Gopalsamy, R.G.; Shanmugam, S.; Serafini, M.R.; Oliveira, M.R.C.; Silva, B.A.F.; Martins, A.O.B.P.B.; Castro, F.F.; et al. Enhancement of orofacial antinociceptive effect of carvacrol, a monoterpene present in oregano and thyme oils, by β-cyclodextrin inclusion complex in mice. Biomed. Pharmacother. 2016, 84, 454–461. [Google Scholar] [CrossRef]
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of phytochemicals with cyclodextrin derivatives—An insight. J. Biomed. Pharmacother. 2017, 88, 1122–1144. [Google Scholar] [CrossRef]
- Moraes, C.M.; Abrami, P.; Gonçalves, M.M.; Andréo Filho, N.; Fernandes, S.A.; de Paula, E.; Fraceto, L.F. Preparação e caracterização físico-química de complexos de inclusão entre anestésicos locais e hidroxipropil-beta-ciclodextrina. Quím. Nova 2007, 30, 777–784. [Google Scholar] [CrossRef]
- Favacho, H.A.S.; Oliveira, B.R.; Santos, K.C.; Medeiros, B.J.L.; Sousa, P.J.C.; Perazzo, F.F.; Carvalho, J.C.T. Anti-inflammatory and antinociceptive activities of Euterpe oleracea Mart., Arecaceae, oil. Rev. Bras. Farmacogn. 2011, 21, 105–114. [Google Scholar] [CrossRef]
- Pinheiro, J.; Tavares, E.; Silva, S.; Félix Silva, J.; Carvalho, Y.; Ferreira, M.; Araújo, A.; Barbosa, E.; Fernandes Pedrosa, M.; Soares, L.; et al. Inclusion complexes of copaiba (Copaifera multijuga Hayne) oleoresin and cyclodextrins: Physicochemical characterization and anti-inflammatory activity. Int. J. Mol. Sci. 2017, 18, 2388. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Ma, L.; Mao, Y.; Lipsky, P.E. Suppression of carrageenan-induced inflammation in vivo by an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F. Inflamm. Res. 1999, 48, 139–148. [Google Scholar] [CrossRef]
- Morikawa, K.; Nonaka, M.; Torii, I.; Morikawa, S. Modulatory effect of fosfomycin on acute inflammation in the rat air pouch model. Int. J. Antimicrob. Agents 2003, 21, 334–339. [Google Scholar] [CrossRef]
- Fusi, J.; Bianchi, S.; Daniele, S.; Pellegrini, S.; Martini, C.; Galetta, F.; Giovannini, L.; Franzoni, F. An in vitro comparative study of the antioxidant activity and SIRT1 modulation of natural compounds. Biomed. Pharmacother. 2018, 101, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.A.L.; Canniatti-Brazaca, S.G. Quantificação de vitamina C e capacidade antioxidante de variedades cítricas. Food Sci. Technol. 2010, 30, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Losada-Barreiro, S.; Bravo-Díaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed]
- Londhe, J.S.; Devasagayam, T.P.A.; Foo, L.Y.; Ghaskadbi, S.S. Radioprotective properties of polyphenols from Phyllanthus amarus Linn. J. Radiat. Res. 2009, 50, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from acai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef] [PubMed]
- Jullian, C.; Orosteguis, T.; Pérez-Cruz, F.; Sánchez, P.; Mendizabal, F.; Olea-Azar, C. Complexation of morin with three kinds of cyclodextrin. Acta A Mol. Biomol. Spectrosc. 2008, 71, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Pu, H.; Tang, P.; Tang, B.; Sun, Q.; Li, H. Propyl gallate/cyclodextrin supramolecular complexes with enhanced solubility and radical scavenging capacity. Food Chem. 2018, 245, 1062–1069. [Google Scholar] [CrossRef]
- Chen, M.; Wu, Q.; Jiang, J.; Jin, X.; Liu, S.; Wang, M.; Zhao, C. Preparation, characterization and in vivo evaluation of a formulation of dantrolene sodium with hydroxypropyl-β-cyclodextrin. J. Pharm. Biomed. Anal. 2017, 135, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, J.; Ma, X.; Huang, G. Inclusion complex of tamibarotene with hydroxypropyl-β-cyclodextrin: Preparation, characterization, in-vitro and in-vivo evaluation. Asian J. Pharm. Sci. 2017, 12, 187–192. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Martins, A.O.B.P.B.; Ribeiro-Filho, J.; Cesário, F.R.A.S.; Castro, F.F.E.; de Albuquerque, T.R.; Fernandes, M.N.M.; da Silva, B.A.F.; Quintans Júnior, L.J.; de Araújo, A.A.S.; et al. Anti-inflammatory activity of the essential oil obtained from Ocimum basilicum complexed with β-cyclodextrin (β-CD) in mice. Food Chem. Toxicol. 2017, 109, 836–846. [Google Scholar] [CrossRef]
- Gaut, J.P.; Yeh, G.C.; Tran, H.D.; Byun, J.; Henderson, J.P.; Richter, G.M.; Brennan, M.-L.; Lusis, A.J.; Belaaouaj, A.; Hotchkiss, R.S.; et al. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc. Natl. Acad. Sci. USA 2001, 98, 11961–11966. [Google Scholar] [CrossRef] [Green Version]
- Van der Veen, B.S.; de Winther, M.P.J.; Heeringa, P. Myeloperoxidase: Molecular mechanisms of action and their relevance to human health and disease. Antioxid. Redox Signal. 2009, 11, 2899–2937. [Google Scholar] [CrossRef]
- Arnhold, J. Properties, functions, and secretion of human myeloperoxidase. Biochemistry 2004, 69, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, M.A.; Pervin, M.; Cha, K.M.; Kim, S.K.; Lim, B.O. Anti-inflammatory activity on mice of extract of Ganoderma lucidum grown on rice via modulation of MAPK and NF-κB pathways. Phytochemistry 2015, 114, 125–136. [Google Scholar] [CrossRef]
- De Moura, R.S.; Ferreira, T.S.; Lopes, A.A.; Pires, K.M.P.; Nesi, R.T.; Resende, A.C.; Souza, P.J.C.; da Silva, A.J.R.; Borges, R.M.; Porto, L.C.; et al. Effects of Euterpe oleracea Mart. (açaí) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine 2012, 19, 262–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bompart, G.J.; Prévot, D.S.; Bascands, J.-L. Rapid automated analysis of glutathione reductase, peroxidase, and S-transferase activity: Application to cisplatin-induced toxicity. Clin. Biochem. 1990, 25, 501–504. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consolaro, A.; Varnier, G.C.; Martini, A.; Ravelli, A. Advances in biomarkers for paediatric rheumatic diseases. Nat. Rev. Rheumatol. 2014, 11, 265–275. [Google Scholar] [CrossRef]
- Poluha, R.L.; Grossmann, E. Inflammatory mediators related to arthrogenic temporomandibular dysfunctions. Br. J. Pain 2018, 1, 60–65. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Pro- versus anti-inflammatory cytokines: Myth or reality. Cell. Mol. Biol. 2001, 47, 695–702. [Google Scholar] [PubMed]
- Presa, F.; Marques, M.; Viana, R.; Nobre, L.; Costa, L.; Rocha, H. The protective role of sulfated polysaccharides from green seaweed Udotea flabellum in cells exposed to oxidative damage. Mar. Drugs 2018, 16, 135. [Google Scholar] [CrossRef] [Green Version]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Yoon, S.; Kwon, Y.; Kim, H.; Roh, D.; Kang, S.; Kim, C.; Han, H.; Kim, K.; Yang, I.; Beitz, A. Intrathecal neostigmine reduces the zymosan-induced inflammatory response in a mouse air pouch model via adrenomedullary activity: Involvement of spinal muscarinic type 2 receptors. Neuropharmacology 2005, 49, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida Magalhães, T.S.S.; de Oliveira Macedo, P.C.; da Costa, É.C.P.; de Aragão Tavares, E.; da Silva, V.C.; Guerra, G.C.B.; Pereira, J.R.; de Araújo Moura Lemos, T.M.; de Negreiros, M.M.F.; de Oliveira Rocha, H.A.; et al. Increase in the Antioxidant and Anti-Inflammatory Activity of Euterpe oleracea Martius Oil Complexed in β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin. Int. J. Mol. Sci. 2021, 22, 11524. https://doi.org/10.3390/ijms222111524
de Almeida Magalhães TSS, de Oliveira Macedo PC, da Costa ÉCP, de Aragão Tavares E, da Silva VC, Guerra GCB, Pereira JR, de Araújo Moura Lemos TM, de Negreiros MMF, de Oliveira Rocha HA, et al. Increase in the Antioxidant and Anti-Inflammatory Activity of Euterpe oleracea Martius Oil Complexed in β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin. International Journal of Molecular Sciences. 2021; 22(21):11524. https://doi.org/10.3390/ijms222111524
Chicago/Turabian Stylede Almeida Magalhães, Thalita Sévia Soares, Pollyana Cristina de Oliveira Macedo, Érika Cibely Pinheiro da Costa, Emanuella de Aragão Tavares, Valéria Costa da Silva, Gerlane Coelho Bernardo Guerra, Joquebede Rodrigues Pereira, Telma Maria de Araújo Moura Lemos, Marília Medeiros Fernandes de Negreiros, Hugo Alexandre de Oliveira Rocha, and et al. 2021. "Increase in the Antioxidant and Anti-Inflammatory Activity of Euterpe oleracea Martius Oil Complexed in β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin" International Journal of Molecular Sciences 22, no. 21: 11524. https://doi.org/10.3390/ijms222111524
APA Stylede Almeida Magalhães, T. S. S., de Oliveira Macedo, P. C., da Costa, É. C. P., de Aragão Tavares, E., da Silva, V. C., Guerra, G. C. B., Pereira, J. R., de Araújo Moura Lemos, T. M., de Negreiros, M. M. F., de Oliveira Rocha, H. A., Converti, A., & de Lima, Á. A. N. (2021). Increase in the Antioxidant and Anti-Inflammatory Activity of Euterpe oleracea Martius Oil Complexed in β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin. International Journal of Molecular Sciences, 22(21), 11524. https://doi.org/10.3390/ijms222111524