Fluorescence Recognition of Anions Using a Heteroditopic Receptor: Homogenous and Two-Phase Sensing
Abstract
:1. Introduction
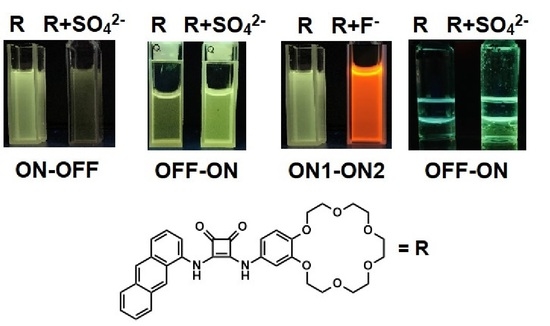
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Synthetic Details
3.3. UV–vis Titration Experiments
3.4. NMR Titration Experiments
3.5. Extraction Experiments
3.6. Emission Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McNaughton, D.A.; Faresa, M.; Picci, G.; Gale, P.A.; Caltagirone, C. Advances in fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2021, 427, 213573. [Google Scholar] [CrossRef]
- Guo, C.H.; Sedgwick, A.C.; Hirao, T.; Sessler, J.L. Supramolecular fluorescent sensors: An historical overview and update. Coord. Chem. Rev. 2021, 427, 213560. [Google Scholar] [CrossRef]
- Evans, N.H.; Beer, P.D. Advances in anion supramolecular chemistry: From recognition to chemical applications. Angew. Chem. Int. Ed. 2014, 53, 11716–11754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busschaert, N.; Caltagirone, C.; Rossom, W.V.; Gale, P.A. Applications of supramolecular anion recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Gale, P.A. From Anion Receptors to Transporters. Acc. Chem. Res. 2011, 44, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.J.; Sarneel, J.M.; Willers, B.J.; Roelofs, J.G.; Verhoeven, J.T.; Lamers, L.P. Interacting effects of sulphate pollution, sulphide toxicity and eutrophication on vegetation development in fens: A mesocosm experiment. Environ. Pollut. 2009, 157, 2072–2081. [Google Scholar] [CrossRef]
- Gale, P.A. Anion and ion-pair receptor chemistry: Highlights from 2000 and 2001. Coord. Chem. Rev. 2003, 240, 3219–3244. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q. Research Advances in Identifying Sulfate Contamination Sources of Water Environment by Using Stable Isotopes. Int. J. Environ. Res. Public Health 2019, 16, 1914. [Google Scholar] [CrossRef] [Green Version]
- Olomu, A.B.; Vickers, C.R.; Waring, R.H.; Clements, D.; Babbs, C.; Warnes, T.W.; Elias, E. High incidence of poor sulphoxidation in patients with PBC. N. Engl. J. Med. 1988, 318, 1089–1092. [Google Scholar] [CrossRef]
- Murch, S.H.; MacDonald, T.T.; Walker-Smith, J.A.; Levin, M.; Lionetti, P.; Klein, N.J. Disruption of sulphated glycosaminoglycans in intestinal inflammation. Lancet 1993, 341, 711–714. [Google Scholar] [CrossRef]
- Amerongen, A.V.N.; Bolscher, J.G.M.; Bloemena, E.; Veerman, E.C.I. Sulfomucins in the human body. Biol. Chem. 1998, 379, 1–26. [Google Scholar]
- Nakanishi, T.; Otaki, Y.; Hasuike, Y.; Nanami, M.; Itahana, R.; Miyagawa, K.; Nishikage, H.; Izumi, M.; Takamitsu, Y. Association of hyperhomocysteinemia with plasma sulfate and urine sulfate excretion in patients with progressive renal disease. Am. J. Kidney Dis. 2002, 40, 909–915. [Google Scholar] [CrossRef]
- Markovich, D. Physiological roles and regulation of mammalian sulfate transporters. Physiol. Rev. 2001, 81, 1499–1533. [Google Scholar] [CrossRef] [PubMed]
- Bader, M. Sulfate removal technologies for oil fields seawater injection operations. J. Pet. Sci. Eng. 2007, 55, 93–110. [Google Scholar] [CrossRef]
- Moyer, B.A.; Custelcean, R.; Hay, B.P.; Sessler, J.L.; Bowman-James, K.; Day, V.W.; Kang, S.O. A Case for Molecular Recognition in Nuclear Separations: Sulfate Separation from Nuclear Wastes. Inorg. Chem. 2013, 52, 3473–3490. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, I.; Ghosh, P. Recognition and separation of sulfate anions. Chem. Soc. Rev. 2012, 41, 3077–3098. [Google Scholar] [CrossRef]
- Katayev, E.A.; Ustynyuk, Y.A.; Sessler, J.L. Molecular recognition of pertechnetate and perrhenate. Coord. Chem. Rev. 2006, 250, 3004–3037. [Google Scholar] [CrossRef]
- Tomas, S.; Prohens, R.; Deslongchamps, G.; Ballester, P.; Costa, A. An effective fluorescent sensor for choline-containing phospholipids. Angew. Chem. Int. Ed. 1999, 38, 2208–2211. [Google Scholar] [CrossRef]
- Elmes, R.B.P.; Turner, P.; Jolliffe, K.A. Colorimetric and luminescent sensors for chloride: Hydrogen bonding vs deprotonation. Org. Lett. 2013, 15, 5638–5641. [Google Scholar] [CrossRef] [Green Version]
- Danao, A.; Ramalingam, V.; Ramamurthy, V.; Muthyala, R.S. On the origin of chloride-induced emission enhancement in ortho substituted squaramides. JPPA Chem. 2017, 344, 108–113. [Google Scholar] [CrossRef]
- Picci, G.; Kubicki, M.; Garau, A.; Lippolis, V.; Mocci, R.; Porcheddu, A.; Quesada, R.; Ricci, P.C.; Scorciapino, M.A.; Caltagirone, C. Simple squaramide receptors for highly efficient anion binding in aqueous media and transmembrane transport. Chem. Commun. 2020, 56, 11066–11069. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, L.K.; Abogunrin, A.A.; Kichham, M.; Pardeshi, J.; Fenelon, O.; Schroeder, M.; Elmes, R.B.P. Bromide through an aggregation-disaggregation approach. Front. Chem. 2019, 7, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picci, G.; Milia, J.; Aragoni, M.C.; Arca, M.; Coles, S.J.; Garau, A.; Lippolis, V.; Montis, R.; Orton, J.B.; Caltagirone, C. Switching-On Fluorescence by Copper (II) and Basic Anions: A Case Study with a Pyrene-Functionalized Squaramide. Molecules 2021, 26, 1301. [Google Scholar] [CrossRef] [PubMed]
- Prohens, R.; Martorell, G.; Ballester, P.; Costa, A. A squaramide fluorescent ensemble for monitoring sulfate in water. Chem. Commun. 2001, 16, 1456–1457. [Google Scholar] [CrossRef]
- Rostami, A.; Wei, C.J.; Guérin, G.; Taylor, M.S. Anion Detection by a Fluorescent Poly(squaramide): Self-Assembly of Anion-Binding Sites by Polymer Aggregation. Angew. Chem. Int. Ed. 2011, 50, 2059–2062. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, S. A fluorescent probe for the selective detection of sulfate ions in water. RSC Adv. 2013, 3, 21856–21862. [Google Scholar] [CrossRef]
- Brunetti, E.; Picron, J.-F.; Flidrova, K.; Bruylants, G.; Bartik, K.; Jabin, I. Fluorescent chemosensors for anions and contact ion pairs with a cavity-based selectivity. J. Org. Chem. 2014, 79, 6179–6188. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, Y.; Li, S.; Lan, J.; You, J. Highly selective fluorescent recognition of sulfate in water by two rigid tetrakisimidazolium macrocycles with peripheral chains. J. Am. Chem. Soc. 2013, 135, 14908–14911. [Google Scholar] [CrossRef] [PubMed]
- Schaly, A.; Belda, R.; García-España, E.; Kubik, S. Selective recognition of sulfate anions by a cyclopeptide-derived receptor in aqueous phosphate buffer. Org. Lett. 2013, 15, 6238–6241. [Google Scholar] [CrossRef]
- Reyheller, C.; Kubik, S. Selective sensing of sulfate in aqueous solution using a fluorescent bis(cyclopeptide). Org. Lett. 2007, 9, 5271–5274. [Google Scholar] [CrossRef]
- Bąk, K.M.; Masłowska, K.; Chmielewski, M.J. Selective turn-on fluorescence sensing of sulfate in aqueous–organic mixtures by an uncharged bis(diamidocarbazole) receptor. Org. Biomol. Chem. 2017, 15, 5968–5975. [Google Scholar] [CrossRef]
- Shumilova, T.A.; Rüffer, T.; Lang, H.; Kataev, E.A. Straightforward design of fluorescent receptors for sulfate: Study of non-covalent interactions contributing to host–guest formation. Chem. Eur. J. 2018, 24, 1500–1504. [Google Scholar] [CrossRef]
- Zaleskaya, M.; Jagleniec, D.; Romański, J. Macrocyclic squaramides as ion pair receptors and fluorescent sensors selective towards sulfates. Dalton Trans. 2021, 50, 3904–3915. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Zúñiga, G.I.; Sessler, J.L. Anion and Ion Pair Recognition under Interfacial Aqueous Conditions. In Comprehensive Supramolecular Chemistry II; Atwood, J.L., Ed.; Elsevier: Oxford, UK, 2017; pp. 161–189. [Google Scholar]
- Jagleniec, D.; Dobrzycki, Ł.; Karbarz, M.; Romański, J. Ion-pair induced supramolecular assembly formation for selective extraction and sensing of potassium sulfate. Chem. Sci. 2019, 10, 9542–9547. [Google Scholar] [CrossRef] [PubMed]
- Zaleskaya, M.; Karbarz, M.; Wilczek, M.; Romański, J. Cooperative transport and selective extraction of sulfates by a squaramide-based ion pair receptor: A case of adaptable selectivity. Inorg. Chem. 2020, 59, 13749–13759. [Google Scholar] [CrossRef]
- Zaleskaya, M.; Dobrzycki, Ł.; Romański, J. Highly efficient, tripodal ion-pair receptors for switching selectivity between acetates and sulfates using solid–liquid and liquid–liquid extractions. Int. J. Mol. Sci. 2020, 21, 9465. [Google Scholar] [CrossRef] [PubMed]
- Jagleniec, D.; Wilczek, M.; Romański, J. Tripodal, Squaramide-Based Ion Pair Receptor for Effective Extraction of Sulfate Salt. Molecules 2021, 26, 2751. [Google Scholar] [CrossRef]
- Ikedu, S.; Nishimura, Y.; Arai, T. Kinetics of Hydrogen Bonding between Anthracene Urea Derivatives and Anions in the Excited State. J. Phys. Chem. A 2011, 115, 8227–8233. [Google Scholar] [CrossRef]
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Fabbrizzi, L.; Mosca, L. The interaction of fluoride with fluorogenic ureas: An ON1–OFF–ON2 response. J. Am. Chem. Soc. 2013, 135, 6345–6355. [Google Scholar] [CrossRef]
- Ośmiałowski, B.; Kolehmainen, E.; Kowalska, M. Bond self-association and complexation with double and triple hydrogen bonding counterparts. Uncommon steric effect on intermolecular interaction. J. Org. Chem. 2021, 77, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Fabbrizzi, L.; Milani, M. The squaramide versus urea contest for anion recognition. Chem. Eur. J. 2010, 16, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Zaleskaya, M.; Jagleniec, D.; Karbarz, M.; Dobrzycki, Ł.; Romański, J. Squaramide based ion pair receptors possessing ferrocene as a signaling unit. Inorg. Chem. Front. 2020, 7, 972–983. [Google Scholar] [CrossRef]







| L | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| KTBACl | 2.07 × 105 | 1.14 × 105 | 2.06 × 105 | 9.24 × 104 |
| KNaCl | 2.45 × 105 | 1.76 × 105 | 1.60 × 105 | 1.13 × 105 |
| KKCl | 3.10 × 105 | 2.23 × 105 | 1.59 × 105 | 1.90 × 105 |
| 1 | 1 +1 Equivalent of K+ | 2 | 2 +1 Equivalent of K+ | |
|---|---|---|---|---|
| Br− | 2.73 × 104 | 4.50 × 104 | 2.16 × 104 | 3.15 × 104 |
| NO2− | 2.89 × 104 | 6.50 × 104 | 2.11 × 104 | 5.66 × 104 |
| NO3− | 1.85 × 104 | 2.56 × 104 | 1.02 × 104 | 1.72 × 104 |
| SO42− | (b) | (b) | (b) | (b) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaleskaya-Hernik, M.; Dobrzycki, Ł.; Karbarz, M.; Romański, J. Fluorescence Recognition of Anions Using a Heteroditopic Receptor: Homogenous and Two-Phase Sensing. Int. J. Mol. Sci. 2021, 22, 13396. https://doi.org/10.3390/ijms222413396
Zaleskaya-Hernik M, Dobrzycki Ł, Karbarz M, Romański J. Fluorescence Recognition of Anions Using a Heteroditopic Receptor: Homogenous and Two-Phase Sensing. International Journal of Molecular Sciences. 2021; 22(24):13396. https://doi.org/10.3390/ijms222413396
Chicago/Turabian StyleZaleskaya-Hernik, Marta, Łukasz Dobrzycki, Marcin Karbarz, and Jan Romański. 2021. "Fluorescence Recognition of Anions Using a Heteroditopic Receptor: Homogenous and Two-Phase Sensing" International Journal of Molecular Sciences 22, no. 24: 13396. https://doi.org/10.3390/ijms222413396
APA StyleZaleskaya-Hernik, M., Dobrzycki, Ł., Karbarz, M., & Romański, J. (2021). Fluorescence Recognition of Anions Using a Heteroditopic Receptor: Homogenous and Two-Phase Sensing. International Journal of Molecular Sciences, 22(24), 13396. https://doi.org/10.3390/ijms222413396