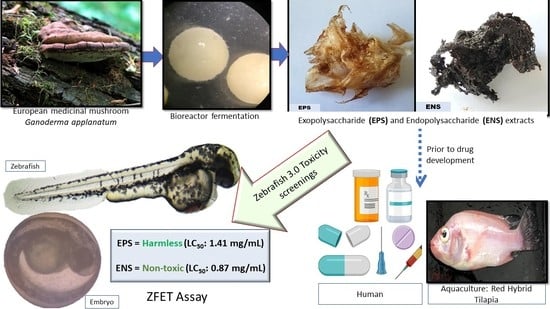
Use of Zebrafish Embryo Assay to Evaluate Toxicity and Safety of Bioreactor-Grown Exopolysaccharides and Endopolysaccharides from European Ganoderma applanatum Mycelium for Future Aquaculture Applications
Abstract
:1. Introduction
2. Results
2.1. Effects of EPS and ENS on the Survival Rate of Zebrafish Embryos
2.2. Performance of EPS-ENS Doses on the Zebrafish Embryos Mortality
2.3. Performance of EPS and ENS Doses on the Zebrafish Embryos Hatching Capability
2.4. The Effects of EPS and ENS on the Heart Rate of Zebrafish Embryos
2.5. Performance of EPS and ENS Doses on the Morphology of Larvae and Zebrafish Embryos Development
3. Discussion
4. Materials and Methods
4.1. European Ganoderma Applanatum Bioreactor Fermentation
4.2. G. applanatum EPS-ENS Preparations
4.3. Zebrafish Maintenance and Breeding
4.4. Zebrafish Embryo Toxicity (ZFET) Assay
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef]
- Liang, Z.; Yuan, Z.; Guo, J.; Wu, J.; Yi, J.; Deng, J.; Shan, Y. Ganoderma lucidum polysaccharides prevent palmitic acid-evoked apoptosis and autophagy in intestinal porcine epithelial cell line via restoration of mitochondrial function and regulation of MAPK and AMPK/Akt/mTOR signaling pathway. Int. J. Mol. Sci. 2019, 20, 478. [Google Scholar] [CrossRef] [Green Version]
- Klaus, A.; Wan-Mohtar, W.A.A.Q.I.; Nikolić, B.; Cvetković, S.; Vunduk, J. Pink oyster mushroom Pleurotus flabellatus mycelium produced by an airlift bioreactor—the evidence of potent in vitro biological activities. World J. Microbiol. Biotechnol. 2021, 37, 1–14. [Google Scholar]
- Wan-Mohtar, W.A.A.Q.I.; Halim-Lim, S.A.; Kamarudin, N.Z.; Rukayadi, Y.; Abd Rahim, M.H.; Jamaludin, A.A.; Ilham, Z. Fruiting-body-base flour from an Oyster mushroom waste in the development of antioxidative chicken patty. J. Food Sci. 2020, 85, 3124–3133. [Google Scholar] [CrossRef]
- Abdullah, N.R.; Sharif, F.; Azizan, N.H.; Hafidz, I.F.M.; Supramani, S.; Usuldin, S.R.A.; Ahmad, R.; Wan-Mohtar, W.A.A.Q.I. Pellet diameter of Ganoderma lucidum in a repeated-batch fermentation for the trio total production of biomass-exopolysaccharide-endopolysaccharide and its anti-oral cancer beta-glucan response. AIMS Microbiol. 2020, 6, 379. [Google Scholar] [CrossRef] [PubMed]
- Roda, E.; De Luca, F.; Di Iorio, C.; Ratto, D.; Siciliani, S.; Ferrari, B.; Cobelli, F.; Borsci, G.; Priori, E.C.; Chinosi, S. Novel Medicinal Mushroom Blend as a Promising Supplement in Integrative Oncology: A Multi-Tiered Study using 4T1 Triple-Negative Mouse Breast Cancer Model. Int. J. Mol. Sci. 2020, 21, 3479. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.A.; Rowan, N.J.; Fogarty, A.M. Novel use of the alga Pseudokirchneriella subcapitata, as an early-warning indicator to identify climate change ambiguity in aquatic environments using freshwater finfish farming as a case study. Sci. Total Environ. 2019, 692, 209–218. [Google Scholar] [CrossRef]
- Tahar, A.; Kennedy, A.; Fitzgerald, R.D.; Clifford, E.; Rowan, N. Full water quality monitoring of a traditional flow-through rainbow trout farm. Fishes 2018, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, E.A.; Stejskal, V.; Clifford, E.; Rowan, N.J. Novel use of peatlands as future locations for the sustainable intensification of freshwater aquaculture production—A case study from the Republic of Ireland. Sci. Total Environ. 2020, 706, 136044. [Google Scholar] [CrossRef]
- Wan-Mohtar, W.A.A.Q.I.; Taufek, N.M.; Yerima, G.; Rahman, J.; Thiran, J.P.; Subramaniam, K.; Sabaratnam, V. Effect of bioreactor-grown biomass from Ganoderma lucidum mycelium on growth performance and physiological response of red hybrid tilapia (Oreochromis sp.) for sustainable aquaculture. Org. Agric. 2020. [Google Scholar] [CrossRef]
- Taufek, N.M.; Harith, H.H.; Abd Rahim, M.H.; Ilham, Z.; Rowan, N.; Wan-Mohtar, W.A.A.Q.I. Performance of mycelial biomass and exopolysaccharide from Malaysian Ganoderma lucidum for the fungivore red hybrid Tilapia (Oreochromis sp.) in Zebrafish embryo. Aquac. Rep. 2020, 17, 100322. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-glucan metabolic and immunomodulatory properties and potential for clinical application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef]
- Supramani, S.; Jailani, N.; Ramarao, K.; Zain, N.A.M.; Klaus, A.; Ahmad, R.; Wan, W.A.A.Q.I. Pellet diameter and morphology of European Ganoderma pfeifferi in a repeated-batch fermentation for exopolysaccharide production. Biocatal. Agric. Biotechnol. 2019, 19, 101118. [Google Scholar] [CrossRef]
- Hassan, N.A.; Supramani, S.; Sohedein, M.N.A.; Usuldin, S.R.A.; Klaus, A.; Ilham, Z.; Chen, W.-H.; Wan-Mohtar, W.A.A.Q.I. Efficient biomass-exopolysaccharide production from an identified wild-Serbian Ganoderma lucidum strain BGF4A1 mycelium in a controlled submerged fermentation. Biocatal. Agric. Biotechnol. 2019, 21, 101305. [Google Scholar] [CrossRef]
- Deborah Paripuranam, T.; Divya, V.; Ulaganathan, P.; Balamurugan, V.; Umamaheswari, S. Replacing fish meal with earthworm and mushroom meals in practical diets of Labeo rohita and Hemigrammus caudovittatus fingerlings. Indian J. Anim. Res. 2011, 45, 115–119. [Google Scholar]
- Baba, E.; Uluköy, G.; Öntaş, C. Effects of feed supplemented with Lentinula edodes mushroom extract on the immune response of rainbow trout, Oncorhynchus mykiss, and disease resistance against Lactococcus garvieae. Aquaculture 2015, 448, 476–482. [Google Scholar] [CrossRef]
- Liang, D.; Yong, T.; Chen, S.; Xie, Y.; Chen, D.; Zhou, X.; Li, D.; Li, M.; Su, L.; Zuo, D. Hypouricemic effect of 2, 5-dihydroxyacetophenone, a computational screened bioactive compound from Ganoderma applanatum, on hyperuricemic mice. Int. J. Mol. Sci. 2018, 19, 1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Zhu, Z.-F.; Cao, L.-P.; Shen, M.; Gao, Y.; Tu, C.-J.; Zhang, Z.-H.; Shan, W.-G. Thermosensitive gel of polysaccharide from Ganoderma applanatum combined with paclitaxel for mice with 4T1 breast cancer. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2020, 45, 2533–2539. [Google Scholar]
- Hanyu, X.; Lanyue, L.; Miao, D.; Wentao, F.; Cangran, C.; Hui, S. Effect of Ganoderma applanatum polysaccharides on MAPK/ERK pathway affecting autophagy in breast cancer MCF-7 cells. Int. J. Biol. Macromol. 2020, 146, 353–362. [Google Scholar] [CrossRef]
- Rahmann, G.; Grimm, D.; Kuenz, A.; Hessel, E. Combining land-based organic and landless food production: A concept for a circular and sustainable food chain for Africa in 2100. Org. Agric. 2020, 10, 9–21. [Google Scholar] [CrossRef]
- Marino, F.; Di Caro, G.; Gugliandolo, C.; Spano, A.; Faggio, C.; Genovese, G.; Morabito, M.; Russo, A.; Barreca, D.; Fazio, F. Preliminary study on the in vitro and in vivo effects of Asparagopsis taxiformis bioactive phycoderivates on teleosts. Front. Physiol. 2016, 7, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Cascio, P.; Calabrò, C.; Bertuccio, C.; Iaria, C.; Marino, F.; Denaro, M.G. Immunohistochemical characterization of PepT1 and ghrelin in gastrointestinal tract of zebrafish: Effects of Spirulina vegetarian diet on the neuroendocrine system cells after alimentary stress. Front. Physiol. 2018, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, A.; Diana, A.; Prosperi, L.; Del Giacco, L. Expression pattern of the small muscle protein, X-linked (smpx) gene during zebrafish embryonic and larval developmental stages. Gene Expr. Patterns 2020, 36, 119110. [Google Scholar] [CrossRef]
- Parolini, M.; Ghilardi, A.; De Felice, B.; Del Giacco, L. Environmental concentration of fluoxetine disturbs larvae behavior and increases the defense response at molecular level in zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2019, 26, 34943–34952. [Google Scholar] [CrossRef]
- Rizzo, C.; Genovese, G.; Morabito, M.; Faggio, C.; Pagano, M.; Spanò, A.; Zammuto, V.; Minicante, S.A.; Manghisi, A.; Cigala, R.M. Potential antibacterial activity of marine macroalgae against pathogens relevant for aquaculture and human health. J. Pure Appl. Microbiol. 2017, 11, 1695–1706. [Google Scholar] [CrossRef]
- Zhang, X.-H.; He, X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Baker, K.; Warren, K.S.; Yellen, G.; Fishman, M.C. Defective “pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc. Natl. Acad. Sci. USA 1997, 94, 4554–4559. [Google Scholar] [CrossRef] [Green Version]
- Kozarski, M.S.; Klaus, A.S.; Vunduk, J.Đ.; Jakovljević, D.M.; Jadranin, M.B.; Nikšić, M.P. Health impact of commercially cultivated mushroom Agaricus bisporus and wild-growing mushroom Ganoderma resinaceum—a comparative overview. J. Serb. Chem. Soc. 2020, 85, 721–735. [Google Scholar] [CrossRef]
- Vunduk, J.; Wan-Mohtar, W.A.A.Q.I.; Mohamad, S.A.; Abd Halim, N.H.; Dzomir, A.Z.M.; Žižak, Ž.; Klaus, A. Polysaccharides of Pleurotus flabellatus strain Mynuk produced by submerged fermentation as a promising novel tool against adhesion and biofilm formation of foodborne pathogens. LWT 2019, 112, 108221. [Google Scholar] [CrossRef]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef]
- Masterson, C.H.; Murphy, E.J.; Gonzalez, H.; Major, I.; McCarthy, S.; O’Toole, D.; Laffey, J.G.; Rowan, N.J. Purified β-glucans from the Shiitake mushroom ameliorates antibiotic-resistant Klebsiella pneumoniae-induced pulmonary sepsis. Lett. Appl. Microbiol. 2020, 71, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Petrovic, P.; Vunduk, J.; Pavlovic, V.; Van Griensven, L.J.L.D. The antimicrobial activities of silver nanoparticles synthesized from medicinal mushrooms. Int. J. Med. Mushrooms 2020, 22, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Jakovljević, D.; Todorović, N.; Wan-Mohtar, W.A.A.Q.I.; Nikšić, M. Ganoderma lucidum as a cosmeceutical: Antiradical potential and inhibitory effect on hyperpigmentation and skin extracellular matrix degradation enzymes. Arch. Biol. Sci. 2019, 71, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Della Torre, C.; Maggioni, D.; Ghilardi, A.; Parolini, M.; Santo, N.; Landi, C.; Madaschi, L.; Magni, S.; Tasselli, S.; Ascagni, M. The interactions of fullerene C60 and Benzo (α) pyrene influence their bioavailability and toxicity to zebrafish embryos. Environ. Pollut. 2018, 241, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Dong, Y.H.; Chen, G.T.; Hu, Q.H. Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr. Polym. 2010, 80, 783–789. [Google Scholar] [CrossRef]
- Ma, Y.; He, H.; Wu, J.; Wang, C.; Chao, K.; Huang, Q. Assessment of Polysaccharides from Mycelia of genus Ganoderma by Mid-Infrared and Near-Infrared Spectroscopy. Sci. Rep. 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, N.; Wan, J.B.; Zhang, D.; Yan, C. Structural characterization and antioxidant activity of a novel heteropolysaccharide from the submerged fermentation mycelia of Ganoderma capense. Carbohydr. Polym. 2015, 134, 752–760. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Dawood, M.A.; Moghadam, M.S.; Sheikhzadeh, N.; Hoseinifar, S.H.; Musthafa, M.S. Modulation of immune parameters and antioxidant defense in zebrafish (Danio rerio) using dietary apple cider vinegar. Aquaculture 2019, 513, 734412. [Google Scholar] [CrossRef]
- Dulay, R.M.R.; Kalaw, S.P.; Reyes, R.G.; Alfonso, N.F.; Eguchi, F. Teratogenic and Toxic Effects of Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (W.Curt.:Fr.) P. Karst. (Higher Basidiomycetes), on Zebrafish Embryo as Model. Int. J. Med. Mushrooms 2012, 14, 507–512. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.E.G.; Dulay, R.M.R.; Enriquez, M. Toxic and Teratogenic Effects of Medicinal and Culinary Mushroom, Termitomyces clypeatus, Collected from the Termite Mound in Mt. Makiling Forest Reserve, Los Baños, Laguna, Philippines on Developing Embryos Of Zebrafish (Danio rerio). Der Pharm. Lett. 2016, 8, 237–242. [Google Scholar]
- Wu, X.; Hao, Q.; Teame, T.; Ding, Q.; Liu, H.; Ran, C.; Yang, Y.; Xia, L.; Wei, S.; Zhou, Z. Gut microbiota induced by dietary GWF® contributes to growth promotion, immune regulation and disease resistance in hybrid sturgeon (Acipenserbaerii x Acipenserschrenckii): Insights from a germ-free zebrafish model. Aquaculture 2020, 520, 734966. [Google Scholar] [CrossRef]
- De Castro, M.; Dulay, R.M.R. Toxic and teratogenic effects of Lentinus sajor-caju and Pleurotus ostreatus ethanolic extracts in Danio rerio embryo model. Int. J. Biol. Pharm. Allied Sci. 2015, 4, 2261–2269. [Google Scholar]
- Gao, Z.; Yuan, F.; Li, H.; Feng, Y.; Zhang, Y.; Zhang, C.; Zhang, J.; Song, Z.; Jia, L. The ameliorations of Ganoderma applanatum residue polysaccharides against CCl4 induced liver injury. Int. J. Biol. Macromol. 2019, 137, 1130–1140. [Google Scholar] [CrossRef]
- Zhen, D.; Su, L.; Miao, Y.; Zhao, F.; Ren, G.; Mahfuz, S.; Song, H. Purification, partial characterization and inducing tumor cell apoptosis activity of a polysaccharide from Ganoderma applanatum. Int. J. Biol. Macromol. 2018, 115, 10–17. [Google Scholar] [CrossRef]
- Mohan, K.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Rajan, D.K. Dietary Ganoderma lucidum polysaccharides to enhance the growth, immune response and disease resistance of freshwater prawn Macrobrachium rosenbergii. Aquac. Rep. 2019, 14, 100203. [Google Scholar] [CrossRef]
- Chithra, E.; Padmanaban, A.; Mohan, K. Potential use of Ganoderma lucidum polysaccharides as a feed supplement in diets on survival and growth performance of the grass carp, Ctenopharyngodon idella. Int. J. Fish. Aquac. Stud. 2016, 4, 328–333. [Google Scholar]
- Wan-Mohtar, W.A.Q.I.; Young, L.; Abbott, G.M.; Clements, C.; Harvey, L.M.; McNeil, B. Antimicrobial properties and cytotoxicity of sulfated (1,3)-beta-D-glucan from the mycelium of the mushroom Ganoderma lucidum. J Microbiol. Biotechnol. 2016, 26, 999–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, W.T.; Lee, S.H.; Dai Kim, J.; Park, Y.S.; Hwang, B.; Lee, S.Y.; Lee, H.Y. Effect of mycelial culture broth of Ganoderma lucidum on the growth characteristics of human cell lines. J. Biosci. Bioeng. 2001, 92, 550–555. [Google Scholar] [CrossRef]
- Supramani, S.; Rahayu Ahmad, Z.I.; Annuar, M.S.M.; Klaus, A.; Wan-Mohtar, W.A.A.Q.I. Optimisation of biomass, exopolysaccharide and intracellular polysaccharide production from the mycelium of an identified Ganoderma lucidum strain QRS 5120 using response surface methodology. AIMS Microbiol. 2019, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Usuldin, S.R.A.; Mahmud, N.; Ilham, Z.; Ikram, N.K.K.; Ahmad, R.; Wan-Mohtar, W.A.A.Q.I. In-depth spectral characterization of antioxidative (1, 3)-β-D-glucan from the mycelium of an identified tiger milk mushroom Lignosus rhinocerus strain ABI in a stirred-tank bioreactor. Biocatal. Agric. Biotechnol. 2020, 23, 101455. [Google Scholar] [CrossRef]
- Nik Ubaidillah, N.H.; Abdullah, N.; Sabaratnam, V. Isolation of the intracellular and extracellular polysaccharides of Ganoderma neojaponicum (Imazeki) and characterization of their immunomodulatory properties. Electron. J. Biotechnol. 2015, 18, 188–195. [Google Scholar] [CrossRef] [Green Version]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Aspatwar, A.; Hammaren, M.M.; Parikka, M.; Parkkila, S. Rapid Evaluation of Toxicity of Chemical Compounds Using Zebrafish Embryos. J. Vis. Exp. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gourmelon, A.; Delrue, N. Validation in support of internationally harmonised OECD test guidelines for assessing the safety of chemicals. Valid. Altern. Methods Toxic. Test. 2016, 856, 9–32. [Google Scholar]
- Parenti, C.C.; Ghilardi, A.; Della Torre, C.; Magni, S.; Del Giacco, L.; Binelli, A. Evaluation of the infiltration of polystyrene nanobeads in zebrafish embryo tissues after short-term exposure and the related biochemical and behavioural effects. Environ. Pollut. 2019, 254, 112947. [Google Scholar] [CrossRef]
- Rowan, N.J.; Galanakis, C.M. Unlocking challenges and opportunities presented by COVID-19 pandemic for cross-cutting disruption in agri-food and green deal innovations: Quo Vadis? Sci. Total Environ. 2020, 748, 141362. [Google Scholar] [CrossRef]







| Source | Toxicological Model | Non-Toxic Concentrations (μg/mL) | References | |
|---|---|---|---|---|
| Exopolysaccharide (EPS) | Endopolysaccharide (ENS) | |||
| G. applanatum BGS6Ap | In vivo—Zebrafish embryos and larvae | 1410 | 870 | Current study |
| G. lucidum QRS 5120 | In vivo—Zebrafish embryos and larvae | 2648 | NA | [11] |
| G. lucidum BCCM 31549 | In vitro—normal human prostate cell line (PN2TA) | 500 | NA | [48] |
| G. lucidum | In vitro—normal human lung cell (WRL68) | 1000 | NA | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan-Mohtar, W.A.A.Q.I.; Ilham, Z.; Jamaludin, A.A.; Rowan, N. Use of Zebrafish Embryo Assay to Evaluate Toxicity and Safety of Bioreactor-Grown Exopolysaccharides and Endopolysaccharides from European Ganoderma applanatum Mycelium for Future Aquaculture Applications. Int. J. Mol. Sci. 2021, 22, 1675. https://doi.org/10.3390/ijms22041675
Wan-Mohtar WAAQI, Ilham Z, Jamaludin AA, Rowan N. Use of Zebrafish Embryo Assay to Evaluate Toxicity and Safety of Bioreactor-Grown Exopolysaccharides and Endopolysaccharides from European Ganoderma applanatum Mycelium for Future Aquaculture Applications. International Journal of Molecular Sciences. 2021; 22(4):1675. https://doi.org/10.3390/ijms22041675
Chicago/Turabian StyleWan-Mohtar, Wan Abd Al Qadr Imad, Zul Ilham, Adi Ainurzaman Jamaludin, and Neil Rowan. 2021. "Use of Zebrafish Embryo Assay to Evaluate Toxicity and Safety of Bioreactor-Grown Exopolysaccharides and Endopolysaccharides from European Ganoderma applanatum Mycelium for Future Aquaculture Applications" International Journal of Molecular Sciences 22, no. 4: 1675. https://doi.org/10.3390/ijms22041675
APA StyleWan-Mohtar, W. A. A. Q. I., Ilham, Z., Jamaludin, A. A., & Rowan, N. (2021). Use of Zebrafish Embryo Assay to Evaluate Toxicity and Safety of Bioreactor-Grown Exopolysaccharides and Endopolysaccharides from European Ganoderma applanatum Mycelium for Future Aquaculture Applications. International Journal of Molecular Sciences, 22(4), 1675. https://doi.org/10.3390/ijms22041675