Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol
Abstract
:1. Introduction
2. Results
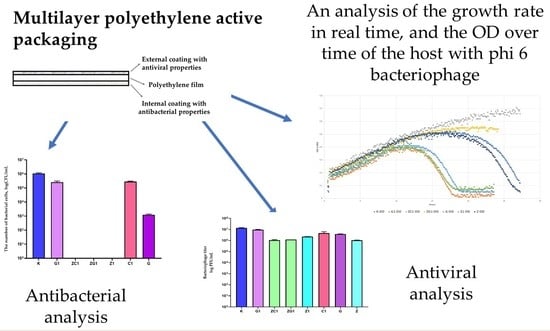
2.1. Antibacterial Analysis
2.2. Antiviral Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Coating Preparation
- (1)
- 4 g of MHPC was introduced into 100 mL of water. The mixture was mixed for 1 h using a magnetic stirrer (Ika, Warsaw, Poland) at 1500 rpm. Then, 95 g of MHPC was mixed with 5 g of geraniol and homogenized (1000 rpm) (Heidolph, Sigma-Aldrich, Poznań, Poland). A mixture of 5% carvacrol in MHPC was not obtained (G).
- (2)
- 8 g of MHPC was introduced into 200 mL of water. The mixture was mixed for 1 h using a magnetic stirrer (Ika, Warsaw, Poland) at 1500 rpm. Then 99.9875 g of MHPC was mixed with 0.0125 g of geraniol (G1), and 99.9875 g of MHPC was mixed with 0.0125 g of carvacrol (C1) (separately) and homogenized (1000 rpm) (Heidolph, Sigma-Aldrich, Poznań, Poland).
- (3)
- 0.082 g of ZnO nanoparticles were introduced into 50 mL of water. As a first step, the mixture was mixed for 1 h using a magnetic stirrer (450 rpm). Next, the mixture was sonicated for 30 min. (sonication parameters: Cycle: 0.5; amplitude: 20%), while at the same time, a 2nd mixture (4 g of MHPC into 50 mL) was prepared as described above. A ZnO nanoparticle solution was introduced into the MHPC mixture (Z) and sonicated for 10 min.
- (4)
- 0.041 g of ZnO nanoparticles were introduced into 50 mL of water. Then, the mixture was mixed for 1 h using a magnetic stirrer (450 rpm). Next, the mixture was sonicated for 30 min (sonication parameters: Cycle: 0.5; amplitude: 20%), while at the same time, the 2nd mixture (4 g of MHPC into 50 mL) was prepared as described above. The ZnO nanoparticle solution was introduced into the MHPC mixture (Z1) and sonicated for 10 min (sonication parameters as described above).
- (5)
- 0.082 g of ZnO nanoparticles were introduced into 100 mL of water. Initially, the mixture was mixed for 1 h using a magnetic stirrer (450 rpm), the mixture was then sonicated for 30 min (sonication parameters: Cycle: 0.5; amplitude: 20%), while at the same time, the 2nd and the 3rd mixtures (99.9875 g of MHPC was mixed with 0.0125 g of geraniol and 99.9875 g of MHPC was mixed with 0.0125 g of carvacrol, separately) were prepared as described above. Then 50 mL of water solution of the nanoparticles was introduced into 50 mL of the 2nd mixture (Z1G1), and 50 mL of nano ZnO solution was introduced into 50 mL of the 3rd mixture (Z1C1). The mixtures were sonicated (sonication parameters: Cycle: 0.5; amplitude: 20%; time: 10 min).
4.3. Antibacterial Analysis
4.4. Antiviral Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akbar, A.; Anal, A.K. Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella Typhimurium and Staphylococcus aureus in readyto-eat poultry meat. Food Control 2014, 38, 88–95. [Google Scholar] [CrossRef]
- Mizielińska, M.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The influence of accelerated UV-A and Q-SUN irradiation on the antimicrobial properties of coatings containing ZnO nanoparticles. Molecules 2017, 22, 1556. [Google Scholar] [CrossRef] [Green Version]
- Mizielińska, M.; Lisiecki, S.; Jotko, M.; Chodzyńska, I.; Bartkowiak, A. The antimicrobial properties of polylactide films covered with ZnO nanoparticles-containing layers. Przem. Chem. 2015, 94, 1000–1003. [Google Scholar]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mat. Sci. Eng. 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Oprea, A.E.; Pandel, L.M.; Dumitrescu, A.M.; Andronescu, E.; Grumezescu, V.; Chifiriuc, M.C.; Mogoantă, L.; Bălşeanu, T.-A.; Mogoşanu, G.D.; Socol, G.; et al. Bioactive ZnO Coatings Deposited by MAPLE—An Appropriate Strategy to Produce Efficient Anti-Biofilm Surfaces. Molecules 2016, 21, 220. [Google Scholar] [CrossRef] [Green Version]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M. Novel active packaging based on carboxymethyl cellulose-chitosan-ZnO NPs nanocomposite for increasing the shelf life of bread. Food Packag. Shelf Life 2017, 11, 106–114. [Google Scholar] [CrossRef]
- Castro-Mayorgaa, J.L.; Fabraa, M.J.; Pourrahimib, A.M.; Olssonb, R.T.; Lagarona, J.M. The impact of zinc oxide particle morphology as anantimicrobial and when incorporated inpoly(3-hydroxybutyrate-co-3-hydroxyvalerate)films for food packaging and food contact surfacesapplications. Food Bioprod. Process. 2017, 101, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lledo, M.L.; Lorente, I. ZnO-PLA nanocomposite coated paper for antimicrobial packaging application. Food Sci. Technol. 2017, 78, 250–257. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.M.A.; Muraleedharan, K. Flexible chitosan-nano ZnO antimicrobial pouches as a new material for extending the shelf life of raw meat. Int. J. Biol. Macromol. 2017, 97, 382–391. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. Part A 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Mizielińska, M.; Kowalska, U.; Jarosz, M.; Sumińska, P. A comparison of the effects of packaging containing nano ZnO or polylysine on the microbial purity and texture of cod (Gadus morhua) fillets. Nanomaterials 2018, 8, 158. [Google Scholar] [CrossRef] [Green Version]
- Antonia Nostro, A.; Papalia, T. Antimicrobial Activity of Carvacrol: Current Progress and Future Prospectives. Recent Pat. Anti-Infect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef]
- Zanetti, M.; Ternus, Z.R.; Dalcanton, F.; de Mello, M.M.J.; de Oliveira, D.; Araujo, P.H.H.; Riella, H.G.; Fiori, M.A. Microbiological Characterization of Pure Geraniol and Comparison with Bactericidal Activity of the Cinnamic Acid in Gram-Positive and GramNegative Bacteria. J. Microb. Biochem. Technol. 2015, 7, 186–193. [Google Scholar]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pagès, J.M.; Amaral, L.; Bolla, J.M. Geraniol Restores Antibiotic Activities against Multidrug-Resistant Isolates from Gram-Negative Species. Antimicrob. Agents. Chemother. 2009, 53, 2209–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkata Mohana, S.; Hemalatha, M.; Kopperia, H.; Ranjitha, I.; Kumara, A.K. SARS-CoV-2 in environmental perspective: Occurrence, persistence, surveillance, inactivation and challenges. Chem. Eng. J. 2021, 405, 126893. [Google Scholar] [CrossRef]
- Elsamadony, M.; Fujii, M.; Miura, T.; Watanabe, T. Possible transmission of viruses from contaminated human feces and sewage: Implications for SARS-CoV-2. Sci. Total Environ. 2021, 755, 142575. [Google Scholar] [CrossRef] [PubMed]
- Patrício Silva, A.L.; Prata, J.C.; Walker, T.R.; Duarte, A.C.; Ouyangd, W.; Barcelòe, D.; Rocha-Santos, T. Increased plastic pollution due to COVID-19 pandemic: Challenges and recommendations. Chem. Eng. J. 2021, 405, 126683. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Nagarajan, S.K.; Veena Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Carol, L.; Lednicky, J.A.; Eiguren-Fernandez, A.; Hering, S.; Fan, Z.H.; Wu, C.Y. Determination of the distribution of infectious viruses in aerosol particles using water-based condensational growth technology and a bacteriophage MS2 model. Aerosol Sci. Tech. 2019, 53, 583–593. [Google Scholar] [CrossRef]
- Turgeon, N.; Toulouse, M.J.; Martel, B.; Moineau, S.; Duchaine, C. Comparison of Five Bacteriophages as Models for Viral Aerosol Studies. Appl. Environ. Microbiol. 2014, 80, 4242–4250. [Google Scholar] [CrossRef] [Green Version]
- Prussin, A.J., II; Schwake, D.O.; Lin, K.; Gallagher, D.L.; Buttling, L.; Marr, L.C. Survival of the Enveloped Virus Phi6 in Droplets as a Function of Relative Humidity, Absolute Humidity and Temperature. Appl. Environ. Microbiol. 2018, 84, e00551-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmailzadeh, H.; Sangpour, P.; Shahraz, F.; Hejazi, J.; Khaksar, R. Effect of nanocomposite packaging containing ZnO on growth of Bacillus subtilis and Enterobacter aerogenes. Mat. Sci. Eng. 2016, 58, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.R.; Gowri, S.; Suresh, J.; Sundrarajan, M. Ionic Liquids Assisted Synthesis of ZnO Nanostructures: Controlled Size, Morphology and Antibacterial Properties. J. Mater. Sci. Technol. 2013, 29, 533–538. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, S.; Kaitha, B.S.; Rajputa, J.; Kaur, M. Synthesis of ZnO nanoparticles using surfactant free in-air and microwave method. Appl. Surf. Sci. 2011, 257, 9661–9672. [Google Scholar] [CrossRef]
- ISO 22196-2011: Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. Available online: https://www.iso.org/standard/54431.html (accessed on 3 August 2011).
- ASTM Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agent(s) in Polymeric or Hydrophobic Materials; E 2180-01; 2002; Available online: https://reference.globalspec.com/standard/4472809/astm-e2180-18 (accessed on 1 May 2018).
- Bhetwal, A.; Maharjan, A.; Shakya, S.; Satyal, D.; Ghimire, S.; Khanal, P.R.; Parajuli, N.P. Isolation of Potential Phages against Multidrug-Resistant Bacterial Isolates: Promising Agents in the Rivers of Kathmandu, Nepal. BioMed Res. Int. 2017, 2017, 3723254. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, N.; Rojas, M.J.; Cruz, G.N.F.; Hung, S.H.; Rohwer, F.; Barr, J.J. Phage on tap–a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ 2016, 4, e2261. [Google Scholar] [CrossRef] [Green Version]
- Skaradzińska, A.; Ochocka, M.; Śliwka, P.; Kuźmińska-Bajora, M.; Skaradziński, G.; Friese, A.; Roschanski, N.; Murugaiyan, J.; Roesler, U. Bacteriophage amplification–A comparison of selected methods. J. Virol. Methods 2020, 282, 113856. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, A. Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. https://doi.org/10.3390/ijms22041717
Mizielińska M, Nawrotek P, Stachurska X, Ordon M, Bartkowiak A. Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol. International Journal of Molecular Sciences. 2021; 22(4):1717. https://doi.org/10.3390/ijms22041717
Chicago/Turabian StyleMizielińska, Małgorzata, Paweł Nawrotek, Xymena Stachurska, Magdalena Ordon, and Artur Bartkowiak. 2021. "Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol" International Journal of Molecular Sciences 22, no. 4: 1717. https://doi.org/10.3390/ijms22041717
APA StyleMizielińska, M., Nawrotek, P., Stachurska, X., Ordon, M., & Bartkowiak, A. (2021). Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol. International Journal of Molecular Sciences, 22(4), 1717. https://doi.org/10.3390/ijms22041717