Gravitational Experimental Platform for Animal Models, a New Platform at ESA’s Terrestrial Facilities to Study the Effects of Micro- and Hypergravity on Aquatic and Rodent Animal Models
Abstract
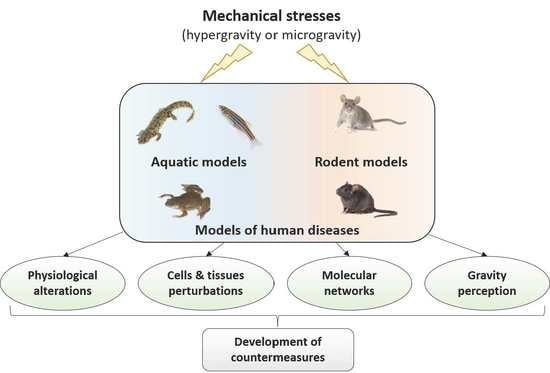
:1. Introduction
2. Equipment Available in the GEPAM Platform
2.1. Aquatic Rotors
2.2. Random Positioning Machine (RPM)
2.3. Rodent Rotor
2.4. Cages for Mouse Hindlimb Unloading
3. Housing Conditions
4. Relevance to ESA’s Space Exploration Program
5. How Can GEPAM Contribute to the Understanding of Molecular and Cellular Mechanisms?
6. How Can GEPAM Contribute to the Identification of Countermeasures?
7. Conclusions and Perspectives
8. Accessibility
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3Rs | Replacement, Reduction and Refinement, a rule for performing more humane animal research |
| ACBS | Animalerie du Campus Biologie Santé (animal facility of the biology and health campus). |
| AHCC | active hexose-correlated compound |
| ESA | European Space Agency |
| g | gravity on Earth |
| GEPAM | gravitational experimental platform for animal models |
| HU | hindlimb unloading |
| ISS | international space station |
| RPM | random positioning machine |
| rVOR | roll-induced vestibulo-ocular reflex |
| StAR-LUE | Structure d’Appui à la Recherche—Lorraine Université d’Excellence (Research Support Structure—Lorraine University of Excellence) |
| TCR | T-cell receptor |
| TLR | Toll-like receptor |
References
- Hariom, S.K.; Ravi, A.; Mohan, G.R.; Pochiraju, H.; Chattopadhyay, S.; Nelson, E. Animal Physiology across the Gravity Continuum. Acta Astronaut. 2021, 178, 522–535. [Google Scholar] [CrossRef]
- English, K.L.; Bloomberg, J.J.; Mulavara, A.P.; Ploutz-Snyder, L.L. Exercise Countermeasures to Neuromuscular Deconditioning in Spaceflight. Compr. Physiol. 2019, 10, 171–196. [Google Scholar] [CrossRef]
- Coupé, M.; Fortrat, J.O.; Larina, I.; Gauquelin-Koch, G.; Gharib, C.; Custaud, M.A. Cardiovascular Deconditioning: From Autonomic Nervous System to Microvascular Dysfunctions. Respir. Physiol. Neurobiol. 2009, 169, S10–S12. [Google Scholar] [CrossRef]
- Fritsch-Yelle, J.M.; Charles, J.B.; Jones, M.M.; Wood, M.L. Microgravity Decreases Heart Rate and Arterial Pressure in Humans. J. Appl. Physiol. 1996, 80, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wan, Y.; Zhang, L.; Tian, Y.; Lv, K.; Li, Y.; Wang, C.; Chen, X.; Chen, S.; Guo, J. Alterations in the Heart Rate and Activity Rhythms of Three Orbital Astronauts on a Space Mission. Life Sci. Space Res. 2015, 4, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Gerbaix, M.; Gnyubkin, V.; Farlay, D.; Olivier, C.; Ammann, P.; Courbon, G.; Laroche, N.; Genthial, R.; Follet, H.; Peyrin, F.; et al. One-Month Spaceflight Compromises the Bone Microstructure, Tissue-Level Mechanical Properties, Osteocyte Survival and Lacunae Volume in Mature Mice Skeletons. Sci. Rep. 2017, 7, 2659. [Google Scholar] [CrossRef] [PubMed]
- Gerbaix, M.; White, H.; Courbon, G.; Shenkman, B.; Gauquelin-Koch, G.; Vico, L. Eight Days of Earth Reambulation Worsen Bone Loss Induced by 1-Month Spaceflight in the Major Weight-Bearing Ankle Bones of Mature Mice. Front Physiol. 2018, 9, 746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vico, L.; Hargens, A. Skeletal Changes during and after Spaceflight. Nat. Rev. Rheumatol. 2018, 14, 229–245. [Google Scholar] [CrossRef]
- Grimm, D.; Grosse, J.; Wehland, M.; Mann, V.; Reseland, J.E.; Sundaresan, A.; Corydon, T.J. The Impact of Microgravity on Bone in Humans. Bone 2016, 87, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Aoki, K.; Masuda, W.; Alles, N.; Nagano, K.; Fukushima, H.; Osawa, K.; Yasuda, H.; Nakamura, I.; Mikuni-Takagaki, Y.; et al. Disruption of NF-ΚB1 Prevents Bone Loss Caused by Mechanical Unloading. J. Bone Miner. Res. 2013, 28, 1457–1467. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Martinez, D.A.; Boudreaux, R.D.; Mantri, A.V. Microgravity Stress: Bone and Connective Tissue. Compr. Physiol. 2016, 6, 645–686. [Google Scholar] [CrossRef]
- Aceto, J.; Nourizadeh-Lillabadi, R.; Marée, R.; Dardenne, N.; Jeanray, N.; Wehenkel, L.; Aleström, P.; van Loon, J.J.W.A.; Muller, M. Zebrafish Bone and General Physiology Are Differently Affected by Hormones or Changes in Gravity. PLoS ONE 2015, 10, e0126928. [Google Scholar] [CrossRef] [Green Version]
- Aceto, J.; Nourizadeh-Lillabadi, R.; Bradamante, S.; Maier, J.A.; Alestrom, P.; van Loon, J.J.; Muller, M. Effects of Microgravity Simulation on Zebrafish Transcriptomes and Bone Physiology-Exposure Starting at 5 Days Post Fertilization. NPJ Microgravity 2016, 2, 16010. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Abe, C.; Tanaka, K. Long-Term Exposure to Microgravity Impairs Vestibulo-Cardiovascular Reflex. Sci. Rep. 2016, 6, 33405. [Google Scholar] [CrossRef]
- Hallgren, E.; Kornilova, L.; Fransen, E.; Glukhikh, D.; Moore, S.T.; Clément, G.; Van Ombergen, A.; MacDougall, H.; Naumov, I.; Wuyts, F.L. Decreased Otolith-Mediated Vestibular Response in 25 Astronauts Induced by Long-Duration Spaceflight. J. Neurophysiol. 2016, 115, 3045–3051. [Google Scholar] [CrossRef] [Green Version]
- Brungs, S.; Hauslage, J.; Hilbig, R.; Hemmersbach, R.; Anken, R. Effects of Simulated Weightlessness on Fish Otolith Growth: Clinostat versus Rotating-Wall Vessel. Adv. Space Res. 2011, 48, 792–798. [Google Scholar] [CrossRef]
- Horn, E.R. Microgravity-Induced Modifications of the Vestibuloocular Reflex in Xenopus Laevis Tadpoles Are Related to Development and the Occurrence of Tail Lordosis. J. Exp. Biol. 2006, 209, 2847–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, M.; Frippiat, J.-P.; Frey, H.; Horn, E.R. The Sensitivity of an Immature Vestibular System to Altered Gravity. J. Exp. Zool A Ecol. Genet. Physiol. 2012, 317, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.; Stowe, R.P.; Mehta, S.; Quiriarte, H.; Pierson, D.; Sams, C. Alterations in Adaptive Immunity Persist during Long-Duration Spaceflight. NPJ Microgravity 2015, 1, 15013. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Feiveson, A.H.; Sams, C.F.; Pierson, D.L. Latent Virus Reactivation in Astronauts on the International Space Station. NPJ Microgravity 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crucian, B.; Babiak-Vazquez, A.; Johnston, S.; Pierson, D.L.; Ott, C.M.; Sams, C. Incidence of Clinical Symptoms during Long-Duration Orbital Spaceflight. Int. J. Gen. Med. 2016, 9, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Crucian, B.; Johnston, S.; Mehta, S.; Stowe, R.; Uchakin, P.; Quiriarte, H.; Pierson, D.; Laudenslager, M.L.; Sams, C. A Case of Persistent Skin Rash and Rhinitis with Immune System Dysregulation Onboard the International Space Station. J. Allergy Clin. Immunol. Pract. 2016, 4, 759–762.e8. [Google Scholar] [CrossRef] [PubMed]
- Guéguinou, N.; Huin-Schohn, C.; Bascove, M.; Bueb, J.-L.; Tschirhart, E.; Legrand-Frossi, C.; Frippiat, J.-P. Could Spaceflight-Associated Immune System Weakening Preclude the Expansion of Human Presence beyond Earth’s Orbit? J. Leukoc. Biol. 2009, 86, 1027–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frippiat, J.-P.; Crucian, B.E.; de Quervain, D.J.-F.; Grimm, D.; Montano, N.; Praun, S.; Roozendaal, B.; Schelling, G.; Thiel, M.; Ullrich, O.; et al. Towards Human Exploration of Space: The THESEUS Review Series on Immunology Research Priorities. NPJ Microgravity 2016, 2, 16040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef]
- Akiyama, T.; Horie, K.; Hinoi, E.; Hiraiwa, M.; Kato, A.; Maekawa, Y.; Takahashi, A.; Furukawa, S. How Does Spaceflight Affect the Acquired Immune System? NPJ Microgravity 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Boxio, R.; Dournon, C.; Frippiat, J.-P. Effects of a Long-Term Spaceflight on Immunoglobulin Heavy Chains of the Urodele Amphibian Pleurodeles xaltl. J. Appl. Physiol. 2005, 98, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Bascove, M.; Huin-Schohn, C.; Guéguinou, N.; Tschirhart, E.; Frippiat, J.-P. Spaceflight-Associated Changes in Immunoglobulin VH Gene Expression in the Amphibian Pleurodeles Waltl. FASEB J. 2009, 23, 1607–1615. [Google Scholar] [CrossRef]
- Bascove, M.; Guéguinou, N.; Schaerlinger, B.; Gauquelin-Koch, G.; Frippiat, J.-P. Decrease in Antibody Somatic Hypermutation Frequency under Extreme, Extended Spaceflight Conditions. FASEB J. 2011, 25, 2947–2955. [Google Scholar] [CrossRef]
- Huin-Schohn, C.; Guéguinou, N.; Schenten, V.; Bascove, M.; Koch, G.G.; Baatout, S.; Tschirhart, E.; Frippiat, J.-P. Gravity Changes during Animal Development Affect IgM Heavy-Chain Transcription and Probably Lymphopoiesis. FASEB J. 2013, 27, 333–341. [Google Scholar] [CrossRef]
- Lescale, C.; Schenten, V.; Djeghloul, D.; Bennabi, M.; Gaignier, F.; Vandamme, K.; Strazielle, C.; Kuzniak, I.; Petite, H.; Dosquet, C.; et al. Hind Limb Unloading, a Model of Spaceflight Conditions, Leads to Decreased B Lymphopoiesis Similar to Aging. FASEB J. 2015, 29, 455–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tascher, G.; Gerbaix, M.; Maes, P.; Chazarin, B.; Ghislin, S.; Antropova, E.; Vassilieva, G.; Ouzren-Zarhloul, N.; Gauquelin-Koch, G.; Vico, L.; et al. Analysis of Femurs from Mice Embarked on Board BION-M1 Biosatellite Reveals a Decrease in Immune Cell Development, Including B Cells, after 1 Wk of Recovery on Earth. FASEB J. 2019, 33, 3772–3783. [Google Scholar] [CrossRef] [PubMed]
- Buchheim, J.-I.; Ghislin, S.; Ouzren, N.; Albuisson, E.; Vanet, A.; Matzel, S.; Ponomarev, S.; Rykova, M.; Choukér, A.; Frippiat, J.-P. Plasticity of the Human IgM Repertoire in Response to Long-Term Spaceflight. FASEB J. 2020, 34, 16144–16162. [Google Scholar] [CrossRef] [PubMed]
- Sachs, L.M.; Buchholz, D.R. Frogs Model Man: In Vivo Thyroid Hormone Signaling during Development. Genesis 2017, 55. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.J.M. Nuclear Receptor Research in Zebrafish. J. Mol. Endocrinol. 2017, 59, R65–R76. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Miller, S.R.; Ober, E.A.; Sadler, K.C. Making It New Again: Insight into Liver Development, Regeneration, and Disease From Zebrafish Research. Curr. Top. Dev. Biol. 2017, 124, 161–195. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, L.J.A.; Philpott, A. Xenopus Models of Cancer: Expanding the Oncologist’s Toolbox. Front. Physiol. 2018, 9, 1660. [Google Scholar] [CrossRef]
- Matsuoka, R.L.; Stainier, D.Y.R. Recent Insights into Vascular Development from Studies in Zebrafish. Curr. Opin. Hematol. 2018, 25, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.M.; Nechiporuk, A.V. Using Zebrafish to Study Collective Cell Migration in Development and Disease. Front. Cell Dev. Biol. 2018, 6, 83. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, A.T.M.; Miller, R.K. Modeling Congenital Kidney Diseases in Xenopus Laevis. Dis. Model. Mech. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Hoppler, S.; Conlon, F.L. Xenopus: Experimental Access to Cardiovascular Development, Regeneration Discovery, and Cardiovascular Heart-Defect Modeling. Cold Spring Harb. Perspect Biol. 2020, 12, a037200. [Google Scholar] [CrossRef] [PubMed]
- Phipps, L.S.; Marshall, L.; Dorey, K.; Amaya, E. Model Systems for Regeneration: Xenopus. Development 2020, 147. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.; Bek, J.W.; Besio, R.; De Clercq, A.; Leoni, L.; Salmon, P.; Coucke, P.J.; Willaert, A.; Forlino, A. Zebrafish: A Resourceful Vertebrate Model to Investigate Skeletal Disorders. Front. Endocrinol. 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Frippiat, J.-P. Contribution of the Urodele Amphibian Pleurodeles Waltl to the Analysis of Spaceflight-Associated Immune System Deregulation. Mol. Immunol. 2013, 56, 434–441. [Google Scholar] [CrossRef]
- Horn, E.; Böser, S.; Franz, M.; Gabriel, M.; Hiesgen, N.; Kuebler, U.; Porciani, M.; Schwarzwälder, A.; Zolesi, V. Development of the Flight Hardware for the Experiment XENOPUS on the Kubik BIO4-Mission. Microgravity Sci. Technol. 2011, 23, 243–248. [Google Scholar] [CrossRef]
- Globus, R.K.; Morey-Holton, E. Hindlimb Unloading: Rodent Analog for Microgravity. J. Appl. Physiol. 2016, 120, 1196–1206. [Google Scholar] [CrossRef]
- Chapes, S.K.; Mastro, A.M.; Sonnenfeld, G.; Berry, W.D. Antiorthostatic Suspension as a Model for the Effects of Spaceflight on the Immune System. J. Leukoc Biol. 1993, 54, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Mouse Genome Informatics. Available online: http://www.informatics.jax.org/ (accessed on 13 March 2021).
- Knight, J. The Zebrafish Information Network. Available online: https://zfin.org/ (accessed on 13 March 2021).
- Karimi, K.; Fortriede, J.D.; Lotay, V.S.; Burns, K.A.; Wang, D.Z.; Fisher, M.E.; Pells, T.J.; James-Zorn, C.; Wang, Y.; Ponferrada, V.G.; et al. Xenbase: A Genomic, Epigenomic and Transcriptomic Model Organism Database. Nucleic Acids Res. 2018, 46, D861–D868. [Google Scholar] [CrossRef]
- Motorine, I. UMS2008/US40 IBSLor. Available online: https://umsibslor.univ-lorraine.fr/en (accessed on 13 March 2021).
- Wang, L.D.; Wagers, A.J. Dynamic Niches in the Origination and Differentiation of Haematopoietic Stem Cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 643–655. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Boudarra, N.; Frippiat, C.; Dournon, C.; Frippiat, J.-P. An Alternative Internal Splicing Site Defines New Ikaros Isoforms in Pleurodeles Waltl. Dev. Comp. Immunol. 2002, 26, 659–673. [Google Scholar] [CrossRef]
- Paulsen, K.; Thiel, C.; Timm, J.; Schmidt, P.M.; Huber, K.; Tauber, S.; Hemmersbach, R.; Seibt, D.; Kroll, H.; Grote, K.-H.; et al. Microgravity-Induced Alterations in Signal Transduction in Cells of the Immune System. Acta Astronaut. 2010, 67, 1116–1125. [Google Scholar] [CrossRef]
- Zwart, S.R.; Pierson, D.; Mehta, S.; Gonda, S.; Smith, S.M. Capacity of Omega-3 Fatty Acids or Eicosapentaenoic Acid to Counteract Weightlessness-Induced Bone Loss by Inhibiting NF-KappaB Activation: From Cells to Bed Rest to Astronauts. J. Bone Miner. Res. 2010, 25, 1049–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, T.T.; Walther, I.; Li, C.-F.; Boonyaratanakornkit, J.; Galleri, G.; Meloni, M.A.; Pippia, P.; Cogoli, A.; Hughes-Fulford, M. The Rel/NF-ΚB Pathway and Transcription of Immediate Early Genes in T Cell Activation Are Inhibited by Microgravity. J. Leukoc. Biol. 2012, 92, 1133–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gridley, D.S.; Mao, X.W.; Stodieck, L.S.; Ferguson, V.L.; Bateman, T.A.; Moldovan, M.; Cunningham, C.E.; Jones, T.A.; Slater, J.M.; Pecaut, M.J. Correction: Changes in Mouse Thymus and Spleen after Return from the STS-135 Mission in Space. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Zhao, T.; Li, R.; Tan, X.; Zhang, J.; Fan, C.; Zhao, Q.; Deng, Y.; Xu, A.; Lukong, K.E.; Genth, H.; et al. Simulated Microgravity Reduces Focal Adhesions and Alters Cytoskeleton and Nuclear Positioning Leading to Enhanced Apoptosis via Suppressing FAK/RhoA-Mediated MTORC1/NF-ΚB and ERK1/2 Pathways. Int. J. Mol. Sci. 2018, 19, 1994. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Moreno-Villanueva, M.; Krieger, S.; Ramesh, G.T.; Neelam, S.; Wu, H. Transcriptomics, NF-ΚB Pathway, and Their Potential Spaceflight-Related Health Consequences. Int. J. Mol. Sci. 2017, 18, 1166. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and Function of NF-KappaB Transcription Factors in the Immune System. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef] [PubMed]
- Guéguinou, N.; Jeandel, J.; Kaminski, S.; Baatout, S.; Ghislin, S.; Frippiat, J.-P. Modulation of Iberian Ribbed Newt Complement Component C3 by Stressors Similar to Those Encountered during a Stay Onboard the International Space Station. Int. J. Mol. Sci. 2019, 20, 1579. [Google Scholar] [CrossRef] [Green Version]
- Buchheim, J.-I.; Matzel, S.; Rykova, M.; Vassilieva, G.; Ponomarev, S.; Nichiporuk, I.; Hörl, M.; Moser, D.; Biere, K.; Feuerecker, M.; et al. Stress Related Shift Toward Inflammaging in Cosmonauts After Long-Duration Space Flight. Front. Physiol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A Multidimensional Analysis of a Year-Long Human Spaceflight. Science 2019, 364. [Google Scholar] [CrossRef]
- Afshinnekoo, E.; Scott, R.T.; MacKay, M.J.; Pariset, E.; Cekanaviciute, E.; Barker, R.; Gilroy, S.; Hassane, D.; Smith, S.M.; Zwart, S.R.; et al. Fundamental Biological Features of Spaceflight: Advancing the Field to Enable Deep-Space Exploration. Cell 2020, 183, 1162–1184. [Google Scholar] [CrossRef]
- da Silveira, W.A.; Fazelinia, H.; Rosenthal, S.B.; Laiakis, E.C.; Kim, M.S.; Meydan, C.; Kidane, Y.; Rathi, K.S.; Smith, S.M.; Stear, B.; et al. Comprehensive Multi-Omics Analysis Reveals Mitochondrial Stress as a Central Biological Hub for Spaceflight Impact. Cell 2020, 183, 1185–1201.e20. [Google Scholar] [CrossRef]
- Zhu, L.; Nie, L.; Xie, S.; Li, M.; Zhu, C.; Qiu, X.; Kuang, J.; Liu, C.; Lu, C.; Li, W.; et al. Attenuation of Antiviral Immune Response Caused by Perturbation of TRIM25-Mediated RIG-I Activation under Simulated Microgravity. Cell Rep. 2021, 34, 108600. [Google Scholar] [CrossRef] [PubMed]
- Bigley, A.B.; Agha, N.H.; Baker, F.L.; Spielmann, G.; Kunz, H.E.; Mylabathula, P.L.; Rooney, B.V.; Laughlin, M.S.; Mehta, S.K.; Pierson, D.L.; et al. NK Cell Function Is Impaired during Long-Duration Spaceflight. J. Appl. Physiol. 2019, 126, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.D.; Alder, M.N. The Evolution of Adaptive Immune Systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonte, C.; Gruez, A.; Ghislin, S.; Frippiat, J.-P. The Urodele Amphibian Pleurodeles Waltl Has a Diverse Repertoire of Immunoglobulin Heavy Chains with Polyreactive and Species-Specific Features. Dev. Comp. Immunol. 2015, 53, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Guéguinou, N.; Huin-Schohn, C.; Ouzren-Zarhloul, N.; Ghislin, S.; Frippiat, J.-P. Molecular Cloning and Expression Analysis of Pleurodeles Waltl Complement Component C3 under Normal Physiological Conditions and Environmental Stresses. Dev. Comp. Immunol. 2014, 46, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, G.; Pagliato, L.; Negroni, M.; Montorfano, G.; Corsetto, P.; Nonnis, S.; Negri, A.; Rizzo, A.M. Protein Pattern of Xenopus Laevis Embryos Grown in Simulated Microgravity. Cell Biol. Int. 2011, 35, 249–258. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Kopp, S.; Wehland, M.; Melnik, D.; Sahana, J.; Krüger, M.; Corydon, T.J.; Oltmann, H.; Schmitz, B.; Schütte, A.; et al. Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3156. [Google Scholar] [CrossRef] [Green Version]
- Thiel, C.S.; Tauber, S.; Lauber, B.; Polzer, J.; Seebacher, C.; Uhl, R.; Neelam, S.; Zhang, Y.; Levine, H.; Ullrich, O. Rapid Morphological and Cytoskeletal Response to Microgravity in Human Primary Macrophages. Int. J. Mol. Sci. 2019, 20, 2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corydon, T.J.; Kopp, S.; Wehland, M.; Braun, M.; Schütte, A.; Mayer, T.; Hülsing, T.; Oltmann, H.; Schmitz, B.; Hemmersbach, R.; et al. Alterations of the Cytoskeleton in Human Cells in Space Proved by Life-Cell Imaging. Sci. Rep. 2016, 6, 20043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Versari, S.; Villa, A.; Bradamante, S.; Maier, J.A.M. Alterations of the Actin Cytoskeleton and Increased Nitric Oxide Synthesis Are Common Features in Human Primary Endothelial Cell Response to Changes in Gravity. Biochim. Biophys. Acta 2007, 1773, 1645–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumei, Y.; Morita, S.; Katano, H.; Akiyama, H.; Hirano, M.; Oyha, K.; Shimokawa, H. Microgravity Signal Ensnarls Cell Adhesion, Cytoskeleton, and Matrix Proteins of Rat Osteoblasts: Osteopontin, CD44, Osteonectin, and Alpha-Tubulin. Ann. N. Y. Acad. Sci. 2006, 1090, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.L. The Cytoskeleton, Apoptosis, and Gene Expression in T Lymphocytes and Other Mammalian Cells Exposed to Altered Gravity. Adv. Space Biol. Med. 2002, 8, 77–128. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D. How Cells (Might) Sense Microgravity. FASEB J. 1999, 13, S3–S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction across the Cell Surface and through the Cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar] [CrossRef]
- Maniotis, A.J.; Chen, C.S.; Ingber, D.E. Demonstration of Mechanical Connections between Integrins, Cytoskeletal Filaments, and Nucleoplasm That Stabilize Nuclear Structure. Proc. Natl. Acad. Sci. USA 1997, 94, 849–854. [Google Scholar] [CrossRef] [Green Version]
- Kawao, N.; Morita, H.; Obata, K.; Tatsumi, K.; Kaji, H. Role of Follistatin in Muscle and Bone Alterations Induced by Gravity Change in Mice. J. Cell Physiol. 2018, 233, 1191–1201. [Google Scholar] [CrossRef]
- Kawao, N.; Morita, H.; Obata, K.; Tamura, Y.; Okumoto, K.; Kaji, H. The Vestibular System Is Critical for the Changes in Muscle and Bone Induced by Hypergravity in Mice. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef]
- Kawao, N.; Morita, H.; Nishida, K.; Obata, K.; Tatsumi, K.; Kaji, H. Effects of Hypergravity on Gene Levels in Anti-Gravity Muscle and Bone through the Vestibular System in Mice. J. Physiol. Sci. 2018, 68, 609–616. [Google Scholar] [CrossRef]
- Ohira, T.; Ino, Y.; Nakai, Y.; Morita, H.; Kimura, A.; Kurata, Y.; Kagawa, H.; Kimura, M.; Egashira, K.; Moriya, S.; et al. Proteomic Analysis Revealed Different Responses to Hypergravity of Soleus and Extensor Digitorum Longus Muscles in Mice. J. Proteom. 2020, 217, 103686. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Zhao, Z.; Ichihara, Y.; Yoshino, D.; Imamura, T.; Sawada, K.; Hayano, S.; Kamioka, H.; Mori, S.; Hirata, H.; et al. Mechanical Regulation of Bone Homeostasis through P130Cas-Mediated Alleviation of NF-ΚB Activity. Sci. Adv. 2019, 5, eaau7802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The Mechanosensitive Piezo1 Channel Is Required for Bone Formation. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical Sensing Protein PIEZO1 Regulates Bone Homeostasis via Osteoblast-Osteoclast Crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayzafoon, M.; Gathings, W.E.; McDonald, J.M. Modeled Microgravity Inhibits Osteogenic Differentiation of Human Mesenchymal Stem Cells and Increases Adipogenesis. Endocrinology 2004, 145, 2421–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoide, T.; Kawao, N.; Tamura, Y.; Morita, H.; Kaji, H. Novel Roles of FKBP5 in Muscle Alteration Induced by Gravity Change in Mice. Biochem. Biophys. Res. Commun. 2016, 479, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Shimoide, T.; Kawao, N.; Morita, H.; Ishida, M.; Takafuji, Y.; Kaji, H. Roles of Olfactomedin 1 in Muscle and Bone Alterations Induced by Gravity Change in Mice. Calcif. Tissue Int. 2020, 107, 180–190. [Google Scholar] [CrossRef]
- Mirzoev, T.; Tyganov, S.; Petrova, I.; Gnyubkin, V.; Laroche, N.; Vico, L.; Shenkman, B. Divergent Anabolic Signalling Responses of Murine Soleus and Tibialis Anterior Muscles to Chronic 2G Hypergravity. Sci. Rep. 2017, 7, 3514. [Google Scholar] [CrossRef] [Green Version]
- Kawao, N.; Morita, H.; Iemura, S.; Ishida, M.; Kaji, H. Roles of Dkk2 in the Linkage from Muscle to Bone during Mechanical Unloading in Mice. Int. J. Mol. Sci. 2020, 21, 2547. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Kong, F.; Liu, C.; Xiao, F.; Dong, X.; Zhang, Y.; Wang, H. Effect of Simulated Microgravity Conditions of Hindlimb Unloading on Mice Hematopoietic and Mesenchymal Stromal Cells. Cell Biol. Int. 2020, 44, 2243–2252. [Google Scholar] [CrossRef]
- Gaignier, F.; Schenten, V.; De Carvalho Bittencourt, M.; Gauquelin-Koch, G.; Frippiat, J.-P.; Legrand-Frossi, C. Three Weeks of Murine Hindlimb Unloading Induces Shifts from B to T and from Th to Tc Splenic Lymphocytes in Absence of Stress and Differentially Reduces Cell-Specific Mitogenic Responses. PLoS ONE 2014, 9, e92664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frett, T.; Petrat, G.; van Loon, J.J.; Hemmersbach, R.; Anken, R. Hypergravity Facilities in the ESA Ground-Based Facility Program–Current Research Activities and Future Tasks. Microgravity Sci. Technol. 2016, 28, 205–214. [Google Scholar] [CrossRef]
- Konishi, T.; Kurazumi, T.; Kato, T.; Takko, C.; Ogawa, Y.; Iwasaki, K.-I. Time-Dependent Changes in Cerebral Blood Flow and Arterial Pressure during Mild+ Gz Hypergravity. Aerosp. Med. Hum. Perform. 2018, 89, 787–791. [Google Scholar] [CrossRef]
- De Cesari, C.; Barravecchia, I.; Pyankova, O.V.; Vezza, M.; Germani, M.M.; Scebba, F.; van Loon, J.J.; Angeloni, D. Hypergravity Activates a Pro-Angiogenic Homeostatic Response by Human Capillary Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnyubkin, V.; Guignandon, A.; Laroche, N.; Vanden-Bossche, A.; Normand, M.; Lafage-Proust, M.-H.; Vico, L. Effects of Chronic Hypergravity: From Adaptive to Deleterious Responses in Growing Mouse Skeleton. J. Appl. Physiol. 2015, 119, 908–917. [Google Scholar] [CrossRef] [Green Version]
- Feuerecker, M.; Crucian, B.E.; Quintens, R.; Buchheim, J.-I.; Salam, A.P.; Rybka, A.; Moreels, M.; Strewe, C.; Stowe, R.; Mehta, S.; et al. Immune Sensitization during 1 Year in the Antarctic High-Altitude Concordia Environment. Allergy 2019, 74, 64–77. [Google Scholar] [CrossRef]
- Jang, T.Y.; Jung, A.-Y.; Kim, Y.H. Hormetic Effect of Chronic Hypergravity in a Mouse Model of Allergic Asthma and Rhinitis. Sci. Rep. 2016, 6, 27260. [Google Scholar] [CrossRef] [Green Version]
- Jang, T.Y.; Jung, A.-Y.; Kwon, S.; Kim, Y.H. Hypergravity Enhances the Therapeutic Effect of Dexamethasone in Allergic Asthma and Rhinitis Animal Model. PLoS ONE 2018, 13, e0197594. [Google Scholar] [CrossRef] [Green Version]
- Dechaumet, B.; Cleret, D.; Linossier, M.-T.; Vanden-Bossche, A.; Chanon, S.; Lefai, E.; Laroche, N.; Lafage-Proust, M.-H.; Vico, L. Hypergravity as a Gravitational Therapy Mitigates the Effects of Knee Osteoarthritis on the Musculoskeletal System in a Murine Model. PLoS ONE 2020, 15, e0243098. [Google Scholar] [CrossRef]
- Guéguinou, N.; Bojados, M.; Jamon, M.; Derradji, H.; Baatout, S.; Tschirhart, E.; Frippiat, J.-P.; Legrand-Frossi, C. Stress Response and Humoral Immune System Alterations Related to Chronic Hypergravity in Mice. Psychoneuroendocrinology 2012, 37, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Taves, M.D.; Hamden, J.E.; Soma, K.K. Local Glucocorticoid Production in Lymphoid Organs of Mice and Birds: Functions in Lymphocyte Development. Horm. Behav. 2017, 88, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Wippert, P.-M.; Rector, M.; Kuhn, G.; Wuertz-Kozak, K. Stress and Alterations in Bones: An Interdisciplinary Perspective. Front. Endocrinol. 2017, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wang, Y.; He, J.; Li, P.; Jin, R.; Wang, K.; Xu, X.; Hao, J.; Zhang, Y.; Liu, H.; et al. Intestinal Microbiota Contributes to Colonic Epithelial Changes in Simulated Microgravity Mouse Model. FASEB J. 2017, 31, 3695–3709. [Google Scholar] [CrossRef] [Green Version]
- Alauzet, C.; Cunat, L.; Wack, M.; Lozniewski, A.; Busby, H.; Agrinier, N.; Cailliez-Grimal, C.; Frippiat, J.-P. Hypergravity Disrupts Murine Intestinal Microbiota. Sci. Rep. 2019, 9, 9410. [Google Scholar] [CrossRef] [PubMed]
- Alauzet, C.; Cunat, L.; Wack, M.; Lanfumey, L.; Legrand-Frossi, C.; Lozniewski, A.; Agrinier, N.; Cailliez-Grimal, C.; Frippiat, J.-P. Impact of a Model Used to Simulate Chronic Socio-Environmental Stressors Encountered during Spaceflight on Murine Intestinal Microbiota. Int. J. Mol. Sci. 2020, 21, 7863. [Google Scholar] [CrossRef] [PubMed]
- Ghislin, S.; Ouzren-Zarhloul, N.; Kaminski, S.; Frippiat, J.-P. Hypergravity Exposure during Gestation Modifies the TCRβ Repertoire of Newborn Mice. Sci. Rep. 2015, 5, 9318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubayle, D.; Vanden-Bossche, A.; Beraneck, M.; Vico, L.; Morel, J.-L. Effects of Centrifugation and Whole-Body Vibrations on Blood-Brain Barrier Permeability in Mice. NPJ Microgravity 2020, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Le, T.T.; Ropars, A.; Sundaresan, A.; Crucian, B.; Choukèr, A.; Frippiat, J.-P. Pharmacological countermeasures to spaceflight-induced alterations of the immune system. In Stress Challenges and Immunity in Space; Choukèr, A., Ed.; Springer: Cham, Switzerland, 2020; pp. 637–657. [Google Scholar]
- Vernikos, J.; Schneider, V.S. Space, Gravity and the Physiology of Aging: Parallel or Convergent Disciplines? A Mini-Review. Gerontology 2010, 56, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Strollo, F.; Gentile, S.; Strollo, G.; Mambro, A.; Vernikos, J. Recent Progress in Space Physiology and Aging. Front. Physiol. 2018, 9, 1551. [Google Scholar] [CrossRef] [Green Version]
- Godbout, J.P.; Glaser, R. Stress-Induced Immune Dysregulation: Implications for Wound Healing, Infectious Disease and Cancer. J. Neuroimmune Pharmacol. 2006, 1, 421–427. [Google Scholar] [CrossRef] [PubMed]
- The European Space Agency. Available online: http://www.esa.int/Science_Exploration/Human_and_Robotic_Exploration/Research/Research_Announcements#GBF (accessed on 13 March 2021).









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnefoy, J.; Ghislin, S.; Beyrend, J.; Coste, F.; Calcagno, G.; Lartaud, I.; Gauquelin-Koch, G.; Poussier, S.; Frippiat, J.-P. Gravitational Experimental Platform for Animal Models, a New Platform at ESA’s Terrestrial Facilities to Study the Effects of Micro- and Hypergravity on Aquatic and Rodent Animal Models. Int. J. Mol. Sci. 2021, 22, 2961. https://doi.org/10.3390/ijms22062961
Bonnefoy J, Ghislin S, Beyrend J, Coste F, Calcagno G, Lartaud I, Gauquelin-Koch G, Poussier S, Frippiat J-P. Gravitational Experimental Platform for Animal Models, a New Platform at ESA’s Terrestrial Facilities to Study the Effects of Micro- and Hypergravity on Aquatic and Rodent Animal Models. International Journal of Molecular Sciences. 2021; 22(6):2961. https://doi.org/10.3390/ijms22062961
Chicago/Turabian StyleBonnefoy, Julie, Stéphanie Ghislin, Jérôme Beyrend, Florence Coste, Gaetano Calcagno, Isabelle Lartaud, Guillemette Gauquelin-Koch, Sylvain Poussier, and Jean-Pol Frippiat. 2021. "Gravitational Experimental Platform for Animal Models, a New Platform at ESA’s Terrestrial Facilities to Study the Effects of Micro- and Hypergravity on Aquatic and Rodent Animal Models" International Journal of Molecular Sciences 22, no. 6: 2961. https://doi.org/10.3390/ijms22062961
APA StyleBonnefoy, J., Ghislin, S., Beyrend, J., Coste, F., Calcagno, G., Lartaud, I., Gauquelin-Koch, G., Poussier, S., & Frippiat, J. -P. (2021). Gravitational Experimental Platform for Animal Models, a New Platform at ESA’s Terrestrial Facilities to Study the Effects of Micro- and Hypergravity on Aquatic and Rodent Animal Models. International Journal of Molecular Sciences, 22(6), 2961. https://doi.org/10.3390/ijms22062961