Multiscale-Engineered Muscle Constructs: PEG Hydrogel Micro-Patterning on an Electrospun PCL Mat Functionalized with Gold Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Fabrication and Characterization of the Basic PCL Scaffold
2.1.1. Coating with Gold Nanoparticles (PCL-Au)
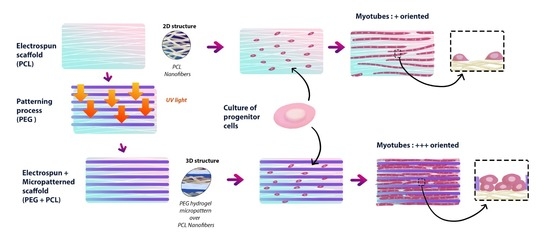
2.1.2. Micropatterning of Multiscale Scaffolds (PCL-PEG and PCL-Au-PEG)
2.1.3. Mechanical Properties of the Different Scaffolds
2.1.4. Influence of Material Composition on Adhesion, Proliferation, and Cell Viability
2.1.5. Cell Differentiation
3. Discussion
4. Materials and Methods
4.1. Scaffold Preparation and Characterization
- Step 1: Preparation of electrospun PCL A solution of 10 wt% poly(ε-caprolactone) (PCL, MW = 80.000 Da, Sigma-Aldrich, St. Louis, MO, USA) in dichloromethane (DCM, Sigma-Aldrich)/N,N-dimethylformamide (DMF, Reagent Plus ≥99%, Sigma-Aldrich) (80:20 v/v) was prepared under stirring for 24 h before electrospinning. Polymer solutions were loaded into a 5 mL syringe equipped with a (18 G) stainless steel gauge needle. Grounded aluminum foil was used as the collector electrode. The distance between needle and aluminum collector was 15 cm and the collector had a diameter of 75 mm. Solution was fed in constantly using a syringe pump at 1.02 mL/h. The voltage applied was optimized to obtain good spinnability, with a typical value of 15 kV.
- Step 2: Preparation of Au NP-doped PCL nanofibrous scaffolds Gold nanoparticles were prepared in surfactant solutions by reduction of HAuCl4. The electrospun scaffolds were immersed in 2 mL of distilled water, successively in a solution of chloroauric acid HAuCl4 (20 mM Sigma-Aldrich). Citrate (70 mM) and ascorbic acid (17.6 mg/mL) were added as reducing agent and stabilizer. The reaction was allowed under stirring for 1 h, during which the color of the scaffolds changed to purple (Supplementary Data S1). Subsequently, the Au NP-doped nanofibrous scaffolds formed were rinsed three times with deionized water and vacuum dried at room temperature for 24 h.
- Step 3: Preparation of (PEG) hydrogel micropatterns on PCL nanofibrous scaffolds The resulting electrospun fibers (with and without Au NPs) were micropatterned with PEG hydrogel using photolithography. PEG-diacrylate (MW 575) was purchased from Sigma-Aldrich. For the UV photo-crosslinking process, the liquid PEG was mixed with 1% w/v of photo-initiator (2-hydroxy-2-methylpropiophenone, Darocur 1173, Sigma Aldrich). Then the mixture was dropped on electrospun scaffolds by spin coating (SPINCOATER model P6700) and exposed to a UV light source for 20 s (Kloé UV-KUB 2, 365 nm, 40 mW/cm) through a photomask. The patterned scaffold was washed carefully in the dark with distilled water to remove the PEG precursor solution.
4.2. Scaffold Conductivity
4.3. Contact Angle Measurement
4.4. Mechanical Properties
4.5. Profilometry
4.6. Cell Seeding on Scaffolds
4.7. Evaluation of Cell Adhesion, Viability, and Proliferation
4.7.1. Adhesion
4.7.2. Viability
4.7.3. Proliferation
4.8. Myotube Measurement
4.9. RT-qPCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNPs | Gold nanoparticles |
| E | Local Young modulus |
| hNSCs | Primary Human Neural Stem Cells |
| MYOG | Myogenin |
| MHC | Myosin heavy chain |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) |
| MYH3 EMB | Myosin heavy chain-embryonic |
| MSTN | Myostatin |
| PCL | Poly(ε-caprolactone) |
| PDMS | Polydimethyl-siloxane |
| PEG | Polyethylene Glycol |
| Sa | Arithmetical mean height |
| Sku | Kurtosis |
| Sp | Maximum peak height |
| Sq | Root mean square height |
| Ssk | Skewness |
| Sv | Maximum pit height |
References
- Juhas, M.; Bursac, N. Engineering skeletal muscle repair. Curr. Opin. Biotechnol. 2013, 24, 880–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shadrin, I.Y.; Khodabukus, A.; Bursac, N. Striated muscle function, regeneration, and repair. Cell. Mol. Life Sci. CMLS 2016, 73, 4175–4202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2014, 96, 183–195. [Google Scholar] [CrossRef]
- Sosa, H.; Popp, D.; Ouyang, G.; Huxley, H. Ultrastructure of skeletal muscle fibers studied by a plunge quick freezing method: Myofilament lengths. Biophys. J. 1994, 67, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Wakelam, M.J. The fusion of myoblasts. Biochem. J. 1985, 228, 1. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Shiwarski, D.J.; Ball, R.L.; Whitehead, K.A.; Feinberg, A.W. Engineering Aligned Skeletal Muscle Tissue Using Decellularized Plant-Derived Scaffolds. ACS Biomater. Sci. Eng. 2020, 6, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- García-Lizarribar, A.; Fernández-Garibay, X.; Velasco-Mallorquí, F.; Castaño, A.G.; Samitier, J.; Ramon-Azcon, J. Composite Biomaterials as Long-Lasting Scaffolds for 3D Bioprinting of Highly Aligned Muscle Tissue. Macromol. Biosci. 2018, 18, 1800167. [Google Scholar] [CrossRef]
- Charest, J.L.; García, A.J.; King, W.P. Myoblast alignment and differentiation on cell culture substrates with microscale topography and model chemistries. Biomaterials 2007, 28, 2202–2210. [Google Scholar] [CrossRef]
- Jiao, A.; Moerk, C.T.; Penland, N.; Perla, M.; Kim, J.; Smith, A.S.T.; Murry, C.E.; Kim, D.H. Regulation of skeletal myotube formation and alignment by nanotopographically controlled cell-secreted extracellular matrix: Regluation of myotube formation by matrix nanotopography. J. Biomed. Mater. Res. Part A 2018, 106, 1543–1551. [Google Scholar] [CrossRef]
- Xu, B.; Magli, A.; Anugrah, Y.; Koester, S.J.; Perlingeiro, R.C.R.; Shen, W. Nanotopography-responsive myotube alignment and orientation as a sensitive phenotypic biomarker for Duchenne Muscular Dystrophy. Biomaterials 2018, 183, 54–66. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef]
- Aviss, K.J.; Gough, J.E.; Downes, S. Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur. Cells Mater. 2010, 19, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, L.; Wang, N.; Gong, S.; Wang, L.; Li, Q.; Shen, C.; Turng, L.S. Fabrication of polycaprolactone electrospun fibers with different hierarchical structures mimicking collagen fibrils for tissue engineering scaffolds. Appl. Surf. Sci. 2018, 427, 311–325. [Google Scholar] [CrossRef]
- Teo, W.E.; He, W.; Ramakrishna, S. Electrospun scaffold tailored for tissue-specific extracellular matrix. Biotechnol. J. 2006, 1, 918–929. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, H.; Yan, J.; Gong, X. Effect of nanofiber orientation of electrospun nanofibrous scaffolds on cell growth and elastin expression of muscle cells. Colloids Surf. Biointerfaces 2015, 136, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Fujita, H.; Nagamori, E. Alignment of skeletal muscle myoblasts and myotubes using linear micropatterned surfaces ground with abrasives. Biotechnol. Bioeng. 2009, 103, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.T.; Sim, S.; Zhu, X.; Takayama, S. The effect of continuous wavy micropatterns on silicone substrates on the alignment of skeletal muscle myoblasts and myotubes. Biomaterials 2006, 27, 4340–4347. [Google Scholar] [CrossRef] [PubMed]
- Junkin, M.; Leung, S.L.; Whitman, S.; Gregorio, C.C.; Wong, P.K. Cellular self-organization by autocatalytic alignment feedback. J. Cell Sci. 2011, 124, 4213–4220. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wu, Y.; Guo, B.; Ma, P.X. Nanofiber Yarn/Hydrogel Core–Shell Scaffolds Mimicking Native Skeletal Muscle Tissue for Guiding 3D Myoblast Alignment, Elongation, and Differentiation. ACS Nano 2015, 9, 9167–9179. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Guo, B.; Ma, P.X. Interwoven Aligned Conductive Nanofiber Yarn/Hydrogel Composite Scaffolds for Engineered 3D Cardiac Anisotropy. ACS Nano 2017, 11, 5646–5659. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Ma, P.X.; Guo, B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.M.; Ribeiro, S.; Ribeiro, C.; Sencadas, V.; Gomes, A.C.; Gama, F.M.; Lanceros-Méndez, S. Effect of poling state and morphology of piezoelectric poly(vinylidene fluoride) membranes for skeletal muscle tissue engineering. RSC Adv. 2013, 3, 17938. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.C.; Lee, J.H.; Jin, L.; Kim, M.J.; Kim, Y.J.; Hyun, J.K.; Jung, T.G.; Hong, S.W.; Han, D.W. Stimulated myoblast differentiation on graphene oxide-impregnated PLGA-collagen hybrid fibre matrices. J. Nanobiotechnol. 2015, 13. [Google Scholar] [CrossRef] [Green Version]
- Mckeon-Fischer, K.D.; Freeman, J.W. Characterization of electrospun poly(L-lactide) and gold nanoparticle composite scaffolds for skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 2010, 5, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Wang, Y.; Su, Y.; Chen, M. 3D myotube guidance on hierarchically organized anisotropic and conductive fibers for skeletal muscle tissue engineering. Mater. Sci. Eng. C 2020, 116, 111070. [Google Scholar] [CrossRef]
- Ahmed, W.W.; Wolfram, T.; Goldyn, A.M.; Bruellhoff, K.; Rioja, B.A.; Möller, M.; Spatz, J.P.; Saif, T.A.; Groll, J.; Kemkemer, R. Myoblast morphology and organization on biochemically micro-patterned hydrogel coatings under cyclic mechanical strain. Biomaterials 2010, 31, 250–258. [Google Scholar] [CrossRef]
- Poinern, G.E.J. Gold Nanoparticle Treated Textile-Based Materials for Potential use as Wearable Sensors. Int. J. Sci. 2016, 2, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.F.; Lee, R.J.; Li, S. Engineering of aligned skeletal muscle by micropatterning. Am. J. Transl. Res. 2010, 2, 43–55. [Google Scholar] [PubMed]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Chen, C.N.; Thompson, L.D.V.; Snow, L.A. Chapter 1—Muscle Structure and Function. In Orthopaedic Physical Therapy Secrets, 3rd ed.; Placzek, J.D., Boyce, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–9. [Google Scholar] [CrossRef]
- Leung, A.F.; Hwang, J.C.; Cheung, Y.M. Determination of myofibrillar diameter by light diffractometry. Pflügers Archiv 1983, 396, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Shumaker-Parry, J.S. Structural Study of Citrate Layers on Gold Nanoparticles: Role of Intermolecular Interactions in Stabilizing Nanoparticles. J. Am. Chem. Soc. 2014, 136, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, C.Y.; Hollister, S.J. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials 2009, 30, 4063–4069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevach, M.; Maoz, B.M.; Feiner, R.; Shapira, A.; Dvir, T. Nanoengineering gold particle composite fibers for cardiac tissue engineering. J. Mater. Chem. B 2013, 1, 5210. [Google Scholar] [CrossRef]
- Rajzer, I.; Rom, M.; Menaszek, E.; Fabia, J.; Kwiatkowski, R. Conductive Polyaniline Patterns on Electrospun Polycaprolactone/Hydroxyapatite Scaffolds for Bone Tissue Engineering. Materials 2021, 14, 4837. [Google Scholar] [CrossRef]
- Ren, K.; Crouzier, T.; Roy, C.; Picart, C. Polyelectrolyte Multilayer Films of Controlled Stiffness Modulate Myoblast Cell Differentiation. Adv. Funct. Mater. 2008, 18, 1378–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogneva, I.V.; Lebedev, D.V.; Shenkman, B.S. Transversal Stiffness and Young’s Modulus of Single Fibers from Rat Soleus Muscle Probed by Atomic Force Microscopy. Biophys. J. 2010, 98, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [Green Version]
- Poveda-Reyes, S.; Moulisova, V.; Sanmartín-Masiá, E.; Quintanilla-Sierra, L.; Salmerón-Sánchez, M.; Ferrer, G.G. Gelatin-Hyaluronic Acid Hydrogels with Tuned Stiffness to Counterbalance Cellular Forces and Promote Cell Differentiation. Macromol. Biosci. 2016, 16, 1311–1324. [Google Scholar] [CrossRef]
- Boontheekul, T.; Hill, E.E.; Kong, H.J.; Mooney, D.J. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007, 13, 1431–1442. [Google Scholar] [CrossRef] [Green Version]
- Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering. Materials 2021, 14, 4773. [Google Scholar] [CrossRef]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly(ε-caprolactone)) Fiber: An Overview. J. Eng. Fibers Fabr. 2014, 9. [Google Scholar] [CrossRef]
- Browning, M.; Cereceres, S.; Luong, P.; Cosgriff-Hernandez, E. Determination of thein vivodegradation mechanism of PEGDA hydrogels. J. Biomed. Mater. Res. Part A 2014, 102, 4244–4251. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Blum, K.M.; Musgrave, A.; Onwuka, E.A.; Yi, T.; Reinhardt, J.W.; A Best, C.; Breuer, C.K. Degradation and in vivo evaluation of polycaprolactone, poly(ε-caprolactone-co-L-lactide), and poly-L-lactic acid as scaffold sealant polymers for murine tissue-engineered vascular grafts. Regen. Med. 2019, 14, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Castellano, D.; Blanes, M.; Marco, B.; Cerrada, I.; Ruiz-Saurí, A.; Pelacho, B.; Araña, M.; Montero, J.A.; Cambra, V.; Prosper, F.; et al. A Comparison of Electrospun Polymers Reveals Poly(3-Hydroxybutyrate)Fiber as a Superior Scaffold for Cardiac Repair. Stem Cells Dev. 2014, 23, 1479–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, M.; Dudhia, J.; Dakin, S.G.; Snelling, S.J.B.; Godoy, R.D.; Mouthuy, P.A.; Smith, R.K.W.; Morrey, M.; Carr, A.J. Histopathological and immunohistochemical evaluation of cellular response to a woven and electrospun polydioxanone (PDO) and polycaprolactone (PCL) patch for tendon repair. Sci. Rep. 2020, 10, 4754. [Google Scholar] [CrossRef] [Green Version]
- Adewale, O.B.; Davids, H.; Cairncross, L.; Roux, S. Toxicological Behavior of Gold Nanoparticles on Various Models: Influence of Physicochemical Properties and Other Factors. Int. J. Toxicol. 2019, 38, 357–384. [Google Scholar] [CrossRef]
- Reid, B.; Gibson, M.; Singh, A.; Taube, J.; Furlong, C.; Murcia, M.; Elisseeff, J. PEG hydrogel degradation and the role of the surrounding tissue environment. J. Tissue Eng. Regen. Med. 2013, 9, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Lynn, A.D.; Blakney, A.K.; Kyriakides, T.R.; Bryant, S.J. Temporal progression of the host response to implanted poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. Part A 2011, 96A, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Altomare, L.; Gadegaard, N.; Visai, L.; Tanzi, M.C.; Farè, S. Biodegradable microgrooved polymeric surfaces obtained by photolithography for skeletal muscle cell orientation and myotube development. Acta Biomater. 2010, 6, 1948–1957. [Google Scholar] [CrossRef]
- Elamparithi, A.; Punnoose, A.M.; Kuruvilla, S.; Ravi, M.; Rao, S.; Paul, S.F. Electrospun polycaprolactone matrices with tensile properties suitable for soft tissue engineering. Artif. Cells Nanomed. Biotechnol. 2016, 44, 878–884. [Google Scholar] [CrossRef]
- Patz, T.M.; Doraiswamy, A.; Narayan, R.J.; Modi, R.; Chrisey, D.B. Two-dimensional differential adherence and alignment of C2C12 myoblasts. Mater. Sci. Eng. B 2005, 123, 242–247. [Google Scholar] [CrossRef]
- Aubin, H.; Nichol, J.W.; Hutson, C.B.; Bae, H.; Sieminski, A.L.; Cropek, D.M.; Akhyari, P.; Khademhosseini, A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 2010, 31, 6941–6951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Li, Q.; Dong, H.; Xiao, W.; Li, L.; Cao, X. Patterning Electrospun Nanofibers via Agarose Hydrogel Stamps to Spatially Coordinate Cell Orientation in Microfluidic Device. Small 2016, 13, 1602610. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jung, H.; Lee, H.R.; Lee, J.S.; Kim, S.R.; Song, K.Y.; Cheong, E.; Bang, J.; Im, S.G.; Cho, S.W. Multiscale, Hierarchically Patterned Topography for Directing Human Neural Stem Cells into Functional Neurons. ACS Nano 2014, 8, 7809–7822. [Google Scholar] [CrossRef] [PubMed]
- Langley, B.; Thomas, M.; Bishop, A.; Sharma, M.; Gilmour, S.; Kambadur, R. Myostatin Inhibits Myoblast Differentiation by Down-regulating MyoD Expression. J. Biol. Chem. 2002, 277, 49831–49840. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, J.M.; García-González, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.; Perlman, H. Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 1997, 7, 597–602. [Google Scholar] [CrossRef]
- Stewart, C.E.H.; James, P.L.; Fant, M.E.; Rotwein, P. Overexpression of insulin-like growth factor-II induces accelerated myoblast differentiation. J. Cell. Physiol. 1996, 169, 23–32. [Google Scholar] [CrossRef]
- Prelle, K.; Wobus, A.M.; Krebs, O.; Blum, W.F.; Wolf, E. Overexpression of Insulin-like Growth Factor-II in Mouse Embryonic Stem Cells Promotes Myogenic Differentiation. Biochem. Biophys. Res. Commun. 2000, 277, 631–638. [Google Scholar] [CrossRef]
- Yang, S.Y.; Goldspink, G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. 2002, 522, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Sacheck, J.M.; Ohtsuka, A.; McLary, S.C.; Goldberg, A.L. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am. J.-Physiol.-Endocrinol. Metab. 2004, 287, E591–E601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, M.; Sharma, A.; Kumar, P.; Kumar, A.; Bharadwaj, A.; Saini, M.; Kardon, G.; Mathew, S.J. Myosin heavy chain-embryonic regulates skeletal muscle differentiation during mammalian development. Development 2020, 147, dev184507. [Google Scholar] [CrossRef] [PubMed]
- Seaborne, R.A.; Hughes, D.C.; Turner, D.C.; Owens, D.J.; Baehr, L.M.; Gorski, P.; Semenova, E.A.; Borisov, O.V.; Larin, A.K.; Popov, D.V.; et al. UBR5 is a novel E3 ubiquitin ligase involved in skeletal muscle hypertrophy and recovery from atrophy. J. Physiol. 2019, 597, 3727–3749. [Google Scholar] [CrossRef] [PubMed]
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beldjilali-Labro, M.; Jellali, R.; Brown, A.D.; Garcia Garcia, A.; Lerebours, A.; Guenin, E.; Bedoui, F.; Dufresne, M.; Stewart, C.; Grosset, J.-F.; et al. Multiscale-Engineered Muscle Constructs: PEG Hydrogel Micro-Patterning on an Electrospun PCL Mat Functionalized with Gold Nanoparticles. Int. J. Mol. Sci. 2022, 23, 260. https://doi.org/10.3390/ijms23010260
Beldjilali-Labro M, Jellali R, Brown AD, Garcia Garcia A, Lerebours A, Guenin E, Bedoui F, Dufresne M, Stewart C, Grosset J-F, et al. Multiscale-Engineered Muscle Constructs: PEG Hydrogel Micro-Patterning on an Electrospun PCL Mat Functionalized with Gold Nanoparticles. International Journal of Molecular Sciences. 2022; 23(1):260. https://doi.org/10.3390/ijms23010260
Chicago/Turabian StyleBeldjilali-Labro, Megane, Rachid Jellali, Alexander David Brown, Alejandro Garcia Garcia, Augustin Lerebours, Erwann Guenin, Fahmi Bedoui, Murielle Dufresne, Claire Stewart, Jean-François Grosset, and et al. 2022. "Multiscale-Engineered Muscle Constructs: PEG Hydrogel Micro-Patterning on an Electrospun PCL Mat Functionalized with Gold Nanoparticles" International Journal of Molecular Sciences 23, no. 1: 260. https://doi.org/10.3390/ijms23010260
APA StyleBeldjilali-Labro, M., Jellali, R., Brown, A. D., Garcia Garcia, A., Lerebours, A., Guenin, E., Bedoui, F., Dufresne, M., Stewart, C., Grosset, J. -F., & Legallais, C. (2022). Multiscale-Engineered Muscle Constructs: PEG Hydrogel Micro-Patterning on an Electrospun PCL Mat Functionalized with Gold Nanoparticles. International Journal of Molecular Sciences, 23(1), 260. https://doi.org/10.3390/ijms23010260