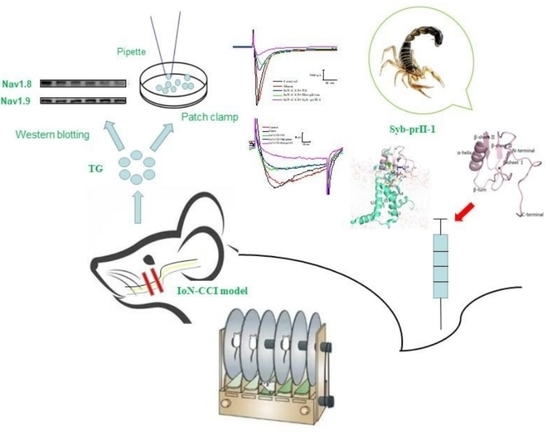
Scorpion Neurotoxin Syb-prII-1 Exerts Analgesic Effect through Nav1.8 Channel and MAPKs Pathway
Abstract
:1. Introduction
2. Results
2.1. Behavioral and Histopathological Changes
2.1.1. Accelerated Rotarod and Gait Analysis
2.1.2. Mechanical Allodynia and Thermal Pain Results
2.1.3. Pathological Changes of the Trigeminal Nerve
2.2. The Expression of Nav1.8 and Nav1.9 in TG Neurons
2.3. The Changes of MAPKs Pathway
2.4. Electrophysiological Results
2.4.1. Effect of Syb-prII-1 on Nav1.8 and Nav1.9 Current Amplitude of TG Neurons
2.4.2. I-V Curve and Steady-State Activation Curve of Nav1.8 and Nav1.9 Currents
2.4.3. Steady-State Inactivation Curves of Nav1.8 and Nav1.9
2.5. The Result of Homology Modeling and Molecular Docking of Syb-prII-1 and VSD2rNav1.8
2.6. The Result of Molecular Dynamics Simulation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. IoN-CCI Model
4.3. Behavioral Testing
4.3.1. Mechanical Allodynia
4.3.2. Thermal Withdrawal Latency
4.3.3. Autonomous Behaviors of Pain
4.3.4. Fatigue Rotating Rod Experiment
4.4. Gait Analysis
4.5. HE Staining of Trigeminal Nerve
4.6. Western Blot
4.7. Immunofluorescence of Trigeminal Nerve Tissue
4.8. Patch Clamp
4.9. Homology Modeling and Molecular Docking
4.10. Molecular Dynamics Simulation
4.11. Materials and Regents
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Cruccu, G.; Finnerup, N.B.; Jensen, T.S.; Scholz, J.; Nurmikko, T. Trigeminal neuralgia: New classification and diagnostic grading for practice and research. Neurology 2016, 87, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Tan, M.; He, L.; Ou, X.; Gao, Y. Inhibitory effects of tetramethylpyrazine on pain transmission of trigeminal neuralgia in CCI-ION rats. Brain Res. Bull. 2017, 134, 72. [Google Scholar] [CrossRef]
- Bowsher, D. Trigeminal Neuralgia: An Anatomically Oriented Review; Wiley Subscription Services, Inc., A Wiley Company: Hoboken, NJ, USA, 1997; Volume 10, pp. 409–415. [Google Scholar] [CrossRef]
- Liu, L.; Hu, W.; Liu, N.; Yang, Q.; Luo, E. Osteoporosis in the jawbones: A correlative factor of primary trigeminal neuralgia? Med. Sci. Monit. 2014, 20, 1481–1485. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Rastogi, S.; Kumar, S.; Reddy, M.P.; Chandra, L. Pain in trigeminal neuralgia: Neurophysiology and measurement: A comprehensive review. J. Med. Life 2013, 6, 383. [Google Scholar] [PubMed]
- Vos, B.P.; Strassman, A.M.; Maciewicz, R.J. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J. Neurosci. Off. J. Soc. Neurosci. 1994, 14, 2708–2723. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; You, Z.; Shen, S.; Yang, J.; Lim, G.; Doheny, J.T.; Chen, L.; Zhu, S.; Mao, J. An Improved Rodent Model of Trigeminal Neuropathic Pain by Unilateral Chronic Constriction Injury of Distal Infraorbital Nerve. J. Pain 2017, 18, 899–907. [Google Scholar] [CrossRef]
- Gabriela, T.; Silvia, B.; Serena, M.; Francesco, D.L.; Gaetano, D.S.; Camilla, F.; Mateus, F.R.; Elisabetta, C.; Maddalena, M.I.; Juliano, F. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain A J. Neurol. 2016, 139, 1361. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.S.; Zhang, T.; Zuo, C.X.; Zuo, Z.F.; Li, H.; Wu, S.X.; Wang, W.; Li, Y.Q. An animal model for trigeminal neuralgia bycompression of the trigeminal nerve root with agar. Pain Physician 2013, 42, 1179. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. Sodium Channels in Normal and Pathological Pain. Annu. Rev. Neurosci. 2010, 33, 325–347. [Google Scholar] [CrossRef] [Green Version]
- Dib-Hajj, S.D.; Black, J.A.; Waxman, S.G. Voltage-Gated Sodium Channels: Therapeutic Targets for Pain. Pain Med. 2010, 10, 1260–1269. [Google Scholar] [CrossRef] [Green Version]
- Abe, M.; Kurihara, T.; Han, W.; Shinomiya, K.; Tanabe, T. Changes in expression of voltage-dependent ion channel subunits in dorsal root ganglia of rats with radicular injury and pain. Spine 2002, 27, 1517. [Google Scholar] [CrossRef] [PubMed]
- Sleeper, A.A.; Cummins, T.R.; Dib-Hajj, S.D.; Hormuzdiar, W.; Black, J.A. Changes in Expression of Two Tetrodotoxin-Resistant Sodium Channels and Their Currents in Dorsal Root Ganglion Neurons after Sciatic Nerve Injury but Not Rhizotomy. J. Neurosci. 2000, 20, 7279–7289. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, J.; Wang, Y.; Wang, L.; Wang, X. Changes in the expression of voltage-gated sodium channels Nav1.3, Nav1.7, Nav1.8, and Nav1.9 in rat trigeminal ganglia following chronic constriction injury. Neuroreport 2016, 27, 929. [Google Scholar] [CrossRef]
- Ito, A.; Takeda, M.; Yoshimura, T.; Komatsu, T. Anti-hyperalgesic effects of calcitonin on neuropathic pain interacting with its peripheral receptors. Mol. Pain 2012, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wilson-Gerwing, T.D.; Stucky, C.L.; Mccomb, G.W.; Verge, V. Neurotrophin-3 significantly reduces sodium channel expression linked to neuropathic pain states. Exp. Neurol. 2008, 213, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, M.F.; Honore, P.; Shieh, C.C.; Chapman, M.; Joshi, S.; Zhang, X.F.; Kort, M.; Carroll, W.; Marron, B.; Atkinson, R. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Channels 2007, 104, 8520–8525. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.; Tang, L.; Madge, D.J.; Stevens, E.B. The role of sodium channels in neuropathic pain. Semin. Cell Dev. Biol. 2006, 17, 571–581. [Google Scholar] [CrossRef]
- Kanellopoulos, A.H.; Matsuyama, A. Voltage-gated sodium channels and pain-related disorders. Clin. Sci. 2016, 130, 2257. [Google Scholar] [CrossRef]
- Bennett, D.L.; Clark, A.J.; Huang, J.; Waxman, S.G.; Dib-Hajj, S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019, 99, 1079–1151. [Google Scholar] [CrossRef]
- Di, S.G.; Truini, A.; Cruccu, G. Current and Innovative Pharmacological Options to Treat Typical and Atypical Trigeminal Neuralgia. Drugs 2018, 78, 1433. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Ban, M.; Bai, F.; Chen, J.; Jin, X.; Song, Y. Anti-Nociceptive and Anti-Inflammation Effect Mechanisms of Mutants of Syb-prII, a Recombinant Neurotoxic Polypeptide. Toxins 2019, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Consortium, U.P. UniProt: A hub for protein information. Nucleic Acids Res. 2015, 43, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Martin-Eauclaire, M.F.; Adi-Bessalem, S.; Hammoudi-Triki, D.; Laraba-Djebari, F.; Bougis, P.E. Serotherapy against Voltage-Gated Sodium Channel-Targeting αToxins from Androctonus Scorpion Venom. Toxins 2019, 11, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Li, T.; Rohou, A.; Arthur, C.P.; Tzakoniati, F.; Wong, E.; Estevez, A.; Kugel, C.; Franke, Y.; Chen, J.U. Structural Basis of Nav1.7 Inhibition by a Gating-Modifier Spider Toxin. Cell 2019, 176, 702–715.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Xu, Y.; Wang, F.; Zhao, M.; Hou, X.; Ma, Y.; Jin, Y.; Liu, Y.; Song, Y.; Zhang, J. The roles of conserved aromatic residues (Tyr5 and Tyr42) in interaction of scorpion toxin BmK AGP-SYPU1 with human Nav1.7. Int. J. Biol. Macromol. 2017, 99, 105–111. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, X.; Hou, X.; Sun, J.; Kong, X.; Sun, Y.; Liu, Z.; Ma, Y.; Niu, Y.; Song, Y. A mutant of the BmK antitumor-analgesic peptide exhibits reduced inhibition to hNav1.4 and hNav1.5 channels while retaining analgesic activity. J. Biol. Chem. 2017, 292, 18270–18280. [Google Scholar] [CrossRef] [Green Version]
- Burchiel, K.J. Abnormal impulse generation in focally demyelinated trigeminal roots. J. Neurosurg. 1980, 53, 674. [Google Scholar] [CrossRef]
- Sakai, Y.; Nishijima, Y.; Mikuni, N.; Iwata, N. An experimental model of hyper-irritability in the trigeminal skin field of the rat. Pain 1979, 7, 147–157. [Google Scholar] [CrossRef]
- Lee, I.O.; Whitehead, R.A.; Ries, C.R.; Schwarz, S.K.W.; Puil, E.; MacLeod, B.A. Erratum to: Evaluation of a novel mouse model of intracisternal strychnine-induced trigeminal allodynia. Can. J. Anesth. J. Can. D’anesthésie 2013, 60, 1034. [Google Scholar] [CrossRef] [Green Version]
- Menétrey, D.; Besson, J.M. Electrophysiological characteristics of dorsal horn cells in rats with cutaneous inflammation resulting from chronic arthritis. Pain 1982, 13, 343–364. [Google Scholar] [CrossRef]
- Matak, I.; Rossetto, O.; Lackovi, Z. Botulinum toxin type A selectivity for certain types of pain is associated with capsaicin-sensitive neurons. Pain 2014, 155, 1516–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Activation of extracellular signal-regulated protein kinases 5 in primary afferent neurons contributes to heat and cold hyperalgesia after inflammation. J. Neurochem. 2010, 102, 1614–1624. [CrossRef]
- Lim, E.J.; Jeon, H.J.; Yang, G.Y.; Min, K.L.; Ju, J.S.; Han, S.R.; Dong, K.A. Intracisternal administration of mitogen-activated protein kinase inhibitors reduced mechanical allodynia following chronic constriction injury of infraorbital nerve in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.N.; Sun, L.H.; Min, W.; Min, Y. Research progress of the role and mechanism of extracellular signal-regulated protein kinase 5 (ERK5) pathway in pathological pain. J. Zhejiang Univ. Sci. B 2016, 17, 733. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; Rullmann, J.; Macarthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef]
- Obata, K.; Noguchi, K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004, 74, 2643–2653. [Google Scholar] [CrossRef]
- Ding, D.; Chen, C.-J.; Starke, R.M.; Kano, H.; Lee, J.Y.K.; Mathieu, D.; Feliciano, C.; Rodriguez-Mercado, R.; Almodovar, L.; Grills, I.S.; et al. Risk of Brain Arteriovenous Malformation Hemorrhage Before and After Stereotactic Radiosurgery. Stroke 2019, 50, 1384–1391. [Google Scholar] [CrossRef]
- Dai, Z.F.; Huang, Q.L.; Liu, H.P.; Zhang, W. Efficacy of stereotactic gamma knife surgery and microvascular decompression in the treatment of primary trigeminal neuralgia: A retrospective study of 220 cases from a single center. J. Pain Res. 2016, 9, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Tang, Q.L.; Xu, J.; Wang, S.Y.; Li, S.H. Dehydrocrenatidine Inhibits Voltage-Gated Sodium Channels and Ameliorates Mechanic Allodia in a Rat Model of Neuropathic Pain. Toxins 2019, 11, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Barbosa, C.; Pei, Z.; Xie, W.; Strong, J.A.; Zhang, J.M.; Cummins, T.R. Increased Resurgent Sodium Currents in Nav1.8 Contribute to Nociceptive Sensory Neuron Hyperexcitability Associated with Peripheral Neuropathies. J. Neurosci. 2019, 39, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Adi, T.; Estacion, M.; Schulman, B.; Vernino, S.; Dib-Hajj, S.; Waxman, S. A novel gain-of-function Na1.7 mutation in a carbamazepine-responsive patient with adult-onset painful peripheral neuropathy. Mol. Pain 2018, 14, 1744806918815007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoeijmakers, J.; Faber, C.; Merkies, I.; Waxman, S. Painful peripheral neuropathy and sodium channel mutations. Neurosci. Lett. 2015, 596, 51–59. [Google Scholar] [CrossRef]
- Touska, F.; Turnquist, B.; Vlachova, V.; Reeh, P.; Leffler, A.; Zimmermann, K. Heat-resistant action potentials require TTX-resistant sodium channels Na1.8 and Na1.9. J. Gen. Physiol. 2018, 150, 1125–1144. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wang, Y.; Liu, Y.; Han, H.; Zhang, D.; Fan, X.; Du, X.; Gamper, N.; Zhang, H. Transcriptional Regulation of Voltage-Gated Sodium Channels Contributes to GM-CSF-Induced Pain. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 5222–5233. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Goregoaker, S.; Engler, H.; Zhou, X.; Mark, L.; Crona, J.; Terry, R.; Hunter, J.; Priestley, T. Small interfering RNA-mediated selective knockdown of Na(V)1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience 2007, 146, 812–821. [Google Scholar] [CrossRef]
- Lai, J.; Gold, M.; Kim, C.; Bian, D.; Ossipov, M.; Hunter, J.; Porreca, F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain 2002, 95, 143–152. [Google Scholar] [CrossRef]
- Lolignier, S.; Amsalem, M.; Maingret, F.; Padilla, F.; Gabriac, M.; Chapuy, E.; Eschalier, A.; Delmas, P.; Busserolles, J. Nav1.9 channel contributes to mechanical and heat pain hypersensitivity induced by subacute and chronic inflammation. PLoS ONE 2011, 6, e23083. [Google Scholar] [CrossRef] [Green Version]
- Tibery, D.; Campos, L.; Mourão, C.; Peigneur, S.; E Carvalho, A.; Tytgat, J.; Schwartz, E. Electrophysiological characterization of Tityus obscurus β toxin 1 (To1) on Na-channel isoforms. Biochim. Biophys. Acta Biomembr. 2019, 1861, 142–150. [Google Scholar] [CrossRef]
- Liang, J.; Liu, X.; Pan, M.; Dai, W.; Dong, Z.; Wang, X.; Liu, R.; Zheng, J.; Yu, S. Blockade of Nav1.8 currents in nociceptive trigeminal neurons contributes to anti-trigeminovascular nociceptive effect of amitriptyline. Neuromolecular Med. 2014, 16, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, J.; Ming, H.; Zhang, Y.; Dun, Y.; Zhang, J.; Song, Y. Buthus martensiiInsights into the binding mode and functional components of the analgesic-antitumour peptide from Karsch to human voltage-gated sodium channel 1.7 based on dynamic simulation analysis. J. Biomol. Struct. Dyn. 2020, 38, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Gibson, T.; Higgins, D. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2002, 2.3.1–2.3.22. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Protein Sci. 2016, 86, 2.9.1–2.9.37. [Google Scholar] [CrossRef] [Green Version]
- Colovos, C.; Yeates, T. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. A Publ. Protein Soc. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; Macarthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Dunbrack, R.L. Whatcheck. In Dictionary of Bioinformatics and Computational Biology; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef] [Green Version]
- Hooft, R.; Vriend, G.; Sander, C.; Abola, E. Errors in protein structures. Nature 1996, 381, 272. [Google Scholar] [CrossRef]
- Chen, R.; Li, L.; Weng, Z. ZDOCK: An initial-stage protein-docking algorithm. Proteins 2003, 52, 80–87. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.; Weng, Z. RDOCK: Refinement of rigid-body protein docking predictions. Proteins 2003, 53, 693–707. [Google Scholar] [CrossRef] [Green Version]
- Mja, A.; Tm, D.; Rsb, C.; Sp, A.; Jcsb, C.; Bh, A.; Ela, D. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers—ScienceDirect. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.R.; Snook, I.K.; Megen, W.V. Hydrodynamic interactions in Brownian dynamics: I. Periodic boundary conditions for computer simulations. Phys. A Stat. Mech. Its Appl. 1987, 143, 441–467. [Google Scholar] [CrossRef]
- Oostenbrink, C.; Soares, T.A.; Vegt, N.; Gunsteren, W. Validation of the 53A6 GROMOS force field. Eur. Biophys. J. 2005, 34, 273–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandt, C.; Ash, W.; Tieleman, D. Setting up and running molecular dynamics simulations of membrane proteins. Methods 2007, 41, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Amira, S.; Spangberg, D.; Hermansson, K. Derivation and evaluation of a flexible SPC model for liquid water. Chem. Phys. 2005, 303, 327–334. [Google Scholar] [CrossRef]
- Lemak, A.S.; Balabaev, N.K. On The Berendsen Thermostat. Mol. Simul. 1994, 13, 177–187. [Google Scholar] [CrossRef]
- Petersen, H.G. Accuracy and efficiency of the particle mesh Ewald method. J. Chem. Phys. 1995, 103, 3668–3679. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation—ScienceDirect. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Makarewicz, T.; Kamierkiewicz, R. Molecular dynamics simulation by GROMACS using GUI plugin for PyMOL. J. Chem. Inf. Modeling 2013, 53, 1229–1234. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]











| Regions on Syb-prII-1 | Residues on Syb-prII-1 | Residues on VSD2rNav1.8 | Types of Interaction Bonds |
|---|---|---|---|
| loop between | K10 | Gly105 | Hydrogen bond |
| α-helix and β-sheet I | S12 | Lys104 | Hydrogen bond |
| Leu107 | Hydrogen bond | ||
| L14 | Leu97 | Hydrophobic | |
| Ala101 | Hydrophobic | ||
| Leu107 | Hydrophobic | ||
| α-helix | N17 | Met44 | Hydrogen bond |
| β-turn | Y31 | Pro43 | Pi-Alkyl |
| Y33 | Met44 | Pi-Sulfur | |
| W35 | Met39 | Hydrogen bond | |
| Met37 | Hydrogen bond | ||
| W37 | Leu110 | Hydrogen bond | |
| Glu98 | Hydrogen bond | ||
| Arg114 | Pi-Cation | ||
| Arg114 | Pi-Cation | ||
| L39 | Val109 | Alkyl |
| Parameter | Unit | Definition |
|---|---|---|
| Stride length | cm | A paw spans the length of a specific stride |
| Paw area | cm2 | Paw area recorded at peak ground contact |
| Stance duration | s | Divided into breaking duration and propulsion duration. Breaking duration describes the time required for the animal’s limbs to decelerate and control the paw to touch the ground; Propulsion duration describes the time required for the animal’s limbs to accelerate and accelerate the starting process. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, F.; Song, Y.; Cao, Y.; Ban, M.; Zhang, Z.; Sun, Y.; Feng, Y.; Li, C. Scorpion Neurotoxin Syb-prII-1 Exerts Analgesic Effect through Nav1.8 Channel and MAPKs Pathway. Int. J. Mol. Sci. 2022, 23, 7065. https://doi.org/10.3390/ijms23137065
Bai F, Song Y, Cao Y, Ban M, Zhang Z, Sun Y, Feng Y, Li C. Scorpion Neurotoxin Syb-prII-1 Exerts Analgesic Effect through Nav1.8 Channel and MAPKs Pathway. International Journal of Molecular Sciences. 2022; 23(13):7065. https://doi.org/10.3390/ijms23137065
Chicago/Turabian StyleBai, Fei, Yongbo Song, Yi Cao, Mengqi Ban, Zhenyu Zhang, Yang Sun, Yuan Feng, and Chunli Li. 2022. "Scorpion Neurotoxin Syb-prII-1 Exerts Analgesic Effect through Nav1.8 Channel and MAPKs Pathway" International Journal of Molecular Sciences 23, no. 13: 7065. https://doi.org/10.3390/ijms23137065
APA StyleBai, F., Song, Y., Cao, Y., Ban, M., Zhang, Z., Sun, Y., Feng, Y., & Li, C. (2022). Scorpion Neurotoxin Syb-prII-1 Exerts Analgesic Effect through Nav1.8 Channel and MAPKs Pathway. International Journal of Molecular Sciences, 23(13), 7065. https://doi.org/10.3390/ijms23137065