1. Introduction
Periodontitis is a chronic inflammatory and destructive disease of periodontal tissue. With the occurrence and development of the disease, gingival bleeding, periodontal pocket formation, and periodontal purulent loose teeth may occur; thus, it is considered the main reason for adult tooth loss [
1]. However, the adverse effects of periodontitis are not limited to periodontal supporting tissues. Persistent periodontitis can affect the overall health of the individual and increase the risk of systemic chronic diseases such as diabetes. Indeed, some scholars have found that the incidence of periodontitis in diabetic patients is 2–3 times higher than that in healthy patients [
2,
3]. Poor blood glucose control increases the severity of gingivitis and periodontitis, and, as such, periodontitis is considered the sixth complication of diabetes [
4]. There is a bidirectional relationship between the two chronic diseases, and both have the ability to induce inflammatory responses. Diabetes can promote apoptosis of periodontal membrane fibroblasts and hinder the repair of periodontal tissue. The inflammatory factors produced by periodontitis can cause circulatory inflammation and affect the blood glucose control and the occurrence and development of complications of diabetes [
5]. As one of the systemic risk factors of periodontitis, diabetes not only increases the risk and severity of periodontitis but also affects its treatment and prognosis.
IL-1β is an important multifunctional cytokine, which is closely related to the cause and process of periodontitis and diabetes. In the process of periodontitis, IL-1β promotes fibroblasts to secrete collagenase, interstitial lytic enzyme, and gelatin-degrading enzyme, which leads to matrix degradation, loss of connective tissue, and destruction of periodontal tissue. IL-1β can also stimulate osteoblasts to secrete plasmin and prostaglandin E2 and promote the differentiation of periodontal ligament mesenchymal stem cells into osteoclasts, which leads to increased inflammation and bone absorption [
6]. IL-1ra can block the biological activity of IL-1β by binding to the IL-1 receptor, which reduces the expression of inflammatory factors, such as IL-6 and TNF-α, and inhibits the formation of osteoclasts [
7]. During the occurrence and development of diabetes, elevated levels of glucose and free fatty acids (FFAs) stimulate islet β cells to produce IL-1β [
8]. IL-1β may accelerate its own production by activating Interleukin 1 receptor 1 (IL-1RI), which induces the production of IL-1-dependent cytokines and chemokines [
8,
9]. Studies have shown that under the stimulation of cytokines such as IL-1β, TNF-α, and interferon γ (IFN-γ), inducible nitric oxide synthase (iNOS), which is seldom expressed in the physiological state, can be produced in large quantities and catalyze the synthesis of excessive nitric oxide (NO). High concentrations of NO can be transformed into free radicals with strong cytotoxicity, causing serious damage to β cells and inhibiting insulin secretion [
9]. The mechanisms underlying the protective effect of IL-1ra on pancreatic islets involve its ability to block the stimulation and production of IL-1β and IL-1-dependent cytokines and chemokines induced by glucose, reduce the activity of iNOS in pancreatic islets, prevent excessive production of NO, reduce the cytokine-induced damage to pancreatic islets, and improve the survival and function of pancreatic islets [
9]. However, IL-1ra is a water-soluble protein drug, which has low bioavailability and a short half-life, requiring large doses and multiple injections, all of which will reduce its therapeutic effect [
10]. Therefore, how to reduce the frequency of administration and improve the efficacy has become a major problem.
The controlled release of IL-1ra is ideal to improve the active duration of the drug and maintain the drug concentration in the local periodontal pocket within the effective concentration range. Temperature-sensitive hydrogel can undergo solution-gel phase transition with a change in temperature, and, as a result, has attracted much attention as an injectable biomaterial with excellent properties. Hydrogel is an insoluble polymer with a three-dimensional network structure, which swells in water and stores high volumes of water. Given the volume of water in the hydrogel stereoscopic network structure, the molecular chain can be extended at will, and it has good fluid properties while maintaining its shape. Moreover, as the biochemical properties of hydrogel are similar to those of human tissue, it has good biocompatibility and is widely used in the field of biomedicine [
11,
12,
13]. The purpose of this study is to develop a new injectable thermo-sensitive hydrogel loaded with IL-1ra for the treatment of periodontal inflammation with diabetes, and to verify the anti-inflammatory properties of the hydrogel through cell experiments and animal experiments, and further explore its effect on blood sugar level. The above contents are marked in red in the revised manuscript.
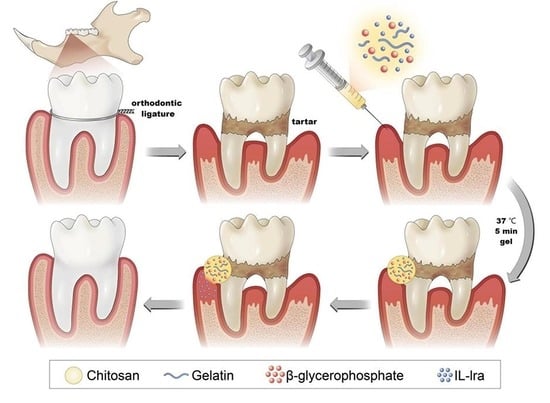
In our research group, chitosan (CS), β-glycerophosphate (β-GP), and gelatin (gel) were mixed at room temperature, and the hydrogel could be changed from the solution to the gel state in approximately 5 min at 37 °C; this duration could be adjusted by changing the ratio of each component to meet the needs of different treatment sites. Moreover, implantation of temperature-sensitive hydrogel into the body for local treatment does not require surgery. The hydrogel should be injected targeting the lesion site through a syringe, thus minimizing the surgical trauma and improving patient comfort. Chitosan-β-glycerophosphate-gelatin temperature-sensitive hydrogel systems can realize drug-sustained release, thus effectively solving the low bioavailability and short half-life of IL-1ra. In previous experiments, we have discussed the effect of IL-1ra on rats with simple periodontitis, so this experiment will not verify it.
3. Discussion
At present, oral hygiene instruction, scaling and subgingival curettage, and scaling and root planing (SRP) are the most basic and effective treatments for periodontal disease. However, affected by dental anatomical conditions such as root-forking lesions and periodontal pocket depth, SRP alone often cannot eliminate plaque microorganisms [
14]. Therefore, local drug therapy has become an important adjunctive therapy for chronic periodontitis. Additionally, for patients with periodontitis with type 2 diabetes, poor blood glucose control will increase periodontal tissue damage and aggravate the development of periodontal disease; this often leads to periodontal inflammation and periodontal tissue trauma caused by prolonged, poor clinical treatment [
3,
4,
5]. CS/β-GP/Gel hydrogel is an injectable temperature-sensitive hydrogel, which has been widely used in the field of tissue engineering as the carrier of seed cells and biological factors. Some scholars have used the chitosan/β-glycerophosphate/collagen (CS/β-GP/Co) hydrogel of tendon-carrying stem cells (TSCs) to promote the healing of acute Achilles tendon injury in rats [
15]. Moreover, injecting CS/β-GP/Gel hydrogel loaded with collagenase into the tendon-bone interface of rabbits can promote the early healing of the tendon-bone interface and the formation of new bone [
16]. In this experiment, three types of raw materials were mixed and formed into a gel at 37 °C, with good morphology and uniform pore size distribution, demonstrating that the hydrogel could realize the sustained release of drugs, and was beneficial to the entry and exit of nutrients and metabolic waste. Comparing the infrared spectra of CS/β-GP/Gel hydrogel and its components, the absorption peak of CS at 3113.4 cm
−1 moves to 2919.9 cm
−1 in the CS/β-GP/Gel at low frequency, which indicates a coordination bond between chitosan and β-GP. Moreover, the formation of a coordination bond changes the electron cloud of the CS amino N, which leads to the weakening of the N-H bond and subsequent stretching vibration and bending vibration. The absorption peaks at 1588.9 cm
−1 and 1498.0 cm
−1 in chitosan hydrogel show that covalent and hydrogen bonds are formed between amino and hydroxyl groups on chitosan and gelatin molecules. XRD showed that the diffraction peaks of the CS and gel were weakened due to the action of -OH and PO43- after β-GP was added, which destroyed the crystalline state of CS. The addition of gelatin led to the formation of a strong hydrogen bond between gelatin and chitosan, weakened the hydrogen bond between -NH2 and -OH in chitosan molecules, and destroyed the ordered structure of chitosan, thus reducing the crystallinity of chitosan and gelatin. The above characterization indicated that CS/β-GP/Gel thermosensitive hydrogel was successfully cross-linked by CS, β-BP, and Gel. Moreover, the in vitro and in vivo studies showed that the hydrogel loaded with IL-1ra CS/β-GP/Gel has good biocompatibility and is a suitable drug carrier.
Although chitosan, as one of the raw materials of CS/β-GP/Gel hydrogels, has hypoglycemic properties, the chitosan used for hydrogels has a large molecular weight (more than 1 million) and poor water solubility, which greatly limits its hypoglycemic effects [
17,
18,
19,
20,
21]. STZ is a cytotoxic glucose analog, which is absorbed by islet β cells through the GLUT2 glucose transporter. After being absorbed into cells, STZ inhibits DNA synthesis by inducing DNA division and methylation, resulting in cell death [
22]. Recent studies have shown that mesenchymal stem cells can reverse the dedifferentiation of islet β cells through IL-1ra, thus alleviating the dysfunction of islet β cells [
23,
24]. Clinical trials have confirmed that IL-1ra can increase insulin secretion by 2.5 times in patients with newly diagnosed type I diabetes [
23]; however, the half-life of IL-1ra is short, only 4–6 h, and the ideal hypoglycemic effect can only be achieved by repeated daily administration at a dosage far higher than our experimental dosage [
10]. Therefore, IL-1ra alone could not effectively reduce blood sugar. Our CS/β-GP/Gel hydrogel maintains the drug concentration in the local periodontal pocket within the effective concentration range for a longer duration. The controlled-release administration mode is ideal to effectively improve the hyperglycemia level of diabetic rats, which is consistent with our experimental results. After treatment with IL-1ra CS/β-GP/Gel hydrogel, the weight loss of diabetic periodontitis rats decreased, possibly due to an improvement in the severity of diabetes promoting weight gain. Furthermore, the decrease in blood glucose level and glycosylated hemoglobin proved that IL-1ra CS/β-GP/Gel hydrogel could improve the hyperglycemia level in diabetic rats.
Periodontitis is a multifactorial disease in which dental plaque is the initial factor. Plaque can induce early inflammatory processes, while local factors, such as poor oral hygiene and occlusal trauma, and systemic factors, such as endocrine disorders and genetic factors, affect the occurrence and development of periodontitis [
2,
25]. The components of dental plaque biofilm can stimulate host cells to produce proinflammatory cytokines, such as IL-1β and TNF-α [
26,
27,
28]. IL-1β is a potential stimulator of osteoclast proliferation, differentiation, and activation, which can induce interstitial cells to produce proteases, including metalloproteinases (MMPs), which are involved in connective tissue destruction [
29,
30,
31,
32]. TNF-α also mediates a series of biological processes and can induce the destruction of connective tissue and alveolar bone [
33]. These biological processes include stimulating bone resorption, inhibiting bone formation, inhibiting proteoglycan synthesis, inducing collagen and cartilage to degrade metalloproteinase and prostaglandin E2, and further producing TNF and other pro-inflammatory cytokines. IL-6 also plays a key role in the initial and acute stages of periodontitis [
34]. In addition to its role in the immune response, IL-6 participates in alveolar bone homeostasis by increasing the expression of the RANKL receptor activator in osteoblasts, thus further promoting osteoclast differentiation and bone resorption. Researchers initially found that injecting IL-1ra into the knee joint of patients with rheumatoid arthritis can relieve pain [
35]. Later, Nixon et al. [
36] used recombinant IL-1ra for local administration and achieved obvious therapeutic effects in mice and Malaysian arthritis animal models. Moreover, HDAd-IL-1ra inhibited the inflammatory effects of factors such as IL-1β, prevented the development of cartilage injury and synovitis, and achieved good anti-inflammatory effects. Deborah J Gorth et al. [
37] prepared IL-1ra/PLGA microspheres using the double emulsion solvent evaporation method. The results showed that IL-1ra was slowly released after 24 h by a sudden release effect and 20 mg had accumulated on the 35th day. In addition, the mRNA expression of iNOS, ADAMTS-4, MMP-13, IL-1β, IL-6, and toll-like receptor 4 (TLR-4) were significantly inhibited by co-culture with mature nucleus pulposus cells. Moreover, Bo Qiu et al. [
38] successfully prepared IL-1ra hyaluronic acid chitosan microspheres, with no obvious sudden release effect, which released linearly for up to 8 days. ELISA results showed that HA-CS-IL-1ra microspheres effectively inhibited the biological activity of IL-1β by inhibiting the secretion of NO
2− and PGE2. Our previous experiments also proved that IL-1ra-loaded sustained-release microspheres can play a role in the treatment of periodontitis [
39]. However, many studies have shown that the microsphere sustained-release preparation has a sudden release effect, despite the long sustained-release time. The severe mechanical action and physical and chemical conditions, such as the organic solvents in the preparation process, may lead to the denaturation of protein drugs. However, the temperature-sensitive hydrogel loaded with IL-1ra CS/β-GP/Gel used in this experiment can release IL-1ra continuously for up to 21 days, ensuring the sustained inhibition of inflammation. Moreover, PCR showed that the expression of IL-1β, IL-6, and TNF-α in cells decreased significantly after treatment with IL-1ra hydrogel.
Previous studies have shown that ligating the neck of rat teeth with silk thread or ligating wire as a local stimulating factor can lead to the formation of histopathological manifestations similar to those of human periodontitis to varying degrees [
40,
41]. Local wire ligation can cause the accumulation of plaque, soft scale, and tartar, and form a local stimulating environment. Local ligation is simple, and early bone resorption is obvious. However, new bone formation may occur over time, while bone loss may decrease, making the model more suitable for short-term study. Two weeks after ligation, compared to the control group, the rats showed redness and swelling of the gums, and infiltration of neutrophils in the epithelium and connective tissue, indicative of the acute stage of periodontitis. The symptoms were worse at 4 weeks after ligation than at 2 weeks, and the gums showed a severe inflammatory reaction. The redness and swelling of gingival epithelium showed severe erosion and even ulcer formation, and a large number of lymphocytes infiltrated in subepithelial connective tissue; collagen degeneration and destruction and alveolar bone destruction were obvious [
42]. Therefore, we removed the ligature wire 4 weeks after ligating the maxillary first molars of rats to observe the inflammatory reaction in the periodontal tissues and observed the changes in the alveolar bone at 4 weeks. The results of tissue PCR were consistent with those of cells. The expression of IL-1β, IL-6, and TNF-α in the periodontal tissues of the IL-1ra hydrogel treatment group decreased significantly. Moreover, IHC showed that the positive areas of IL-1β, IL-6, and TNF-α in the IL-1ra hydrogel treatment group decreased, as did the IRS, demonstrating the effective inhibition of inflammatory factors in periodontal tissues of diabetic rats. The results of HE and Micro-CT showed that the IL-1ra hydrogel could reduce inflammatory cell infiltration in periodontal tissue and relieve alveolar bone absorption, demonstrating great potential for treating periodontitis.
There are still some limitations in this study. Micro-CT results showed that hydrogel reduces the absorption of alveolar bone. Therefore, we can further explore whether the hydrogel has osteogenic properties by changing the levels of osteoblasts in vivo and in vitro, and further study the potential pathways of anti-inflammation and osteogenesis on this basis, which will further improve this experiment. In addition, previous studies have shown that effective treatment of periodontal inflammation can promote a decrease in glycosylated hemoglobin levels [
5,
43]. Therefore, in our experimental results, the decrease in blood glucose level in rats may also be related to the fact that IL-1ra CS/β-GP/Gel thermosensitive hydrogel can effectively relieve local periodontal inflammation and then affect the whole blood glucose level. However, the reason why IL-1ra-loaded thermosensitive hydrogel lowers blood sugar is still unclear. It may be due to the direct effect of IL-1ra on diabetes, or the indirect reduction in blood sugar due to the reduction in local periodontitis after hydrogel treatment, or that both of the above factors exist at the same time. The above-mentioned related mechanism can also be taken as the research focus. Therefore, this experiment still has great research potential to be further explored.
4. Materials and Methods
4.1. Synthesis of IL-1ra CS/β-GP/Gel Temperature-Sensitive Hydrogel
To synthesize IL-1ra CS/β-GP/Gel temperature-sensitive hydrogel, 1 g CS (molecular weight: 310,000–375,000 Da, Sigma-Aldrich, Munich, Germany) was weighed onto weighing paper (10 cm × 10 cm, Hangzhou Fuyang Chengkun Experimental Instrument Co., Ltd., Hangzhou, China), and irradiated under an ultraviolet lamp for 1 h (30 min per side). Following UV sterilization, the CS was dissolved in 50 mL 0.1 mol/L HCl aqueous solution to prepare a 2% (w/v) CS solution. Next, 5.6 g β-GP was dissolved in 10 mL deionized water and configured with a mass fraction of 56% β-GP solution (including 50 μg/mL IL-1ra). Subsequently, 0.2 g gelatin (molecular weight: 40,000–50,000 Da, Sigma-Aldrich, Munich, Germany) was dissolved in 40 mL deionized water to prepare an aqueous gelatin solution with a mass fraction of 0.5%. The β-GP solution and gelatin solution were filter sterilized using a 0.22 μm disposable filter before use. The CS solution, β-GP solution, and gelatin solution were configured in a 40:8:1 volume ratio. Next, 20 mL CS solution was transferred to a beaker and magnetically stirred for 10 min, before adding 4 mL β-GP solution dropwise in an ice bath. When the solution became turbid, 500 μL gelatin aqueous solution was added and magnetically stirred until the solutions were fully mixed. The pH was adjusted to 7.2 with 0.1 mol/L NaOH to obtain CS/β-GP/Gel temperature sensitive hydrogel loaded with IL-1ra. Next, 2 mL hydrogel was transferred into a sterile ampoule, which was sealed and placed in a constant temperature water bath at 37 °C. The gel time was recorded by the tube inversion method.
4.2. Characterization of CS/β-GP/Gel Thermosensitive Hydrogels
The IL-1ra CS/β-GP/Gel temperature-sensitive hydrogel was pre-frozen at –80 °C for 24 h and then freeze-dried. The microstructure of the temperature-sensitive hydrogel was observed by scanning electron microscopy (SEM; SUPRA 55 SAPPHIRE, Carl Zeiss Jena, Germany). Next, 1 mg of freeze-dried IL-1ra CS/β-GP/Gel-loaded temperature-sensitive hydrogel was mixed with an appropriate amount of Kbr powder and tableted. The structure and composition of the organic functional groups of the temperature-sensitive hydrogel were analyzed by Fourier transform infrared spectrometry (FTIR, Nicolet 560 FTIR spectrometer, Nicolet Instrument Corporation, Madison, WI, USA) in the range of 400–4000 cm−1. The freeze-dried hydrogel samples were crushed with a mortar, and the phase structure of the hydrogel was analyzed by an X-ray diffractometer (Bruker AXS GmbH, Karlsruhe, Germany), with the following working conditions: Cu target Kα radiation; Ni as a filter; tube pressure, 60 kV; working current, 40 mA; scanning range, 5–40°; scanning speed, 6 (°)/min; and resolution, 0.02%.
In order to measure the degradation rate and drug release rate of CS/β-GP/Gel thermosensitive hydrogel in vitro, we accurately measured 2 mL of IL-1ra CS/β-GP/Gel thermosensitive hydrogel with a pipette at 4 °C, put it in a 5 mL centrifuge tube, and incubated in a constant temperature incubator at 37 °C for 3 h to ensure that it can be completely transformed into gel. Then, add 2 mL of SBF into the centrifuge tube, and keep incubating in the incubator at 37 °C, and discard the buffer every seven days. The following is the calculation formula of the degradation rate . W0 is the initial gel dry weight and Wt is the dry weight of gel at the moment of degradation.
In the aseptic environment, the mixed solution of IL-1ra CS/β-GP/Gel before gelation was added to a 6-well plate (2 mL per well), and the plate was incubated in an incubator at 37 °C to fully gel. Following incubation, 8 mL SBF was added to each well and further incubated at 37 °C. Following incubation, 3 mL supernatant was collected from each well at 1 h, 2 h, 3 h, 8 h, 24 h, 48 h, 72 h, and 5, 7, 10, 14, and 21 days after incubation, and the wells were supplemented with an equal volume of SBF solution. According to the standard curve in Enzyme-linked immunosorbent assay (ELISA; Dakewe, Beijing, China), the concentration of IL-1ra in supernatant at each time point was quantitatively analyzed. Then calculate the cumulative release rate according to the following formula: . Fi is the cumulative release rate of the system after the ith sampling, Ci is the drug release concentration at the ith sampling, 3 is the volume of each sampling (unit: mL), and 8 is the total volume of the release system (unit: mL), thus drawing the cumulative release curve.
4.3. Cytocompatibility of IL-1ra CS/β-GP/Gel Temperature-Sensitive Hydrogels
In the aseptic environment, the mixed solution of CS/β-GP/Gel was added to a 6-well plate in a volume of 900 μL per well, and then allowed to fully gel in an incubator at 37 °C. After gelling, 3 mL high-sugar medium was added to each well, and the incubation was continued for 24 h in an incubator at 37 °C to prepare an extract. Following incubation, the extract was filtered with a disposable 0.22 μm filter, and fetal bovine serum (FBS) and penicillin-streptomycin solution were added to prepare the extract. Then, mouse macrophages (RAW264.7, Thermo Fisher Scientific, Waltham, MA, USA), which grow well under incubation conditions of 37 °C, 5% CO2, and 100% humidity, were evenly mixed with the extract, and then inoculated into two 96-well plates at a density of 2 × 103/well and incubated for 24 h until adherent. After 24 h, the original medium was replaced with extracts containing different concentrations of IL-1ra (1, 10, 20, 50, 100, 150, 200 μg/mL, w/v) for 24 h and 48 h. Next, 10 μL of CCK-8 reagent was added to each well, before reacting at 37°C for 2 h. The absorbance (OD) at 450 nm was detected using a microplate reader (RT-6000; Lei Du Life Science and Technology Co, Shenzhen, China), and the cell viability was calculated as follows: Cell survival rate = [(experimental well OD value − blank well OD value)/(control well OD value − blank well OD value)] × 100%. The experiment was repeated thrice.
4.4. IL-1ra CS/β-GP/Gel Thermosensitive Hydrogel Inhibits Inflammatory Cytokine Expression in Macrophages under High Glucose Conditions In Vitro
Lipopolysaccharide is the main component of the cell wall of Gram-negative bacteria and can induce macrophages to produce various pro-inflammatory factors, including IL-1β, IL-6, and TNF-α. During periodontal infection, LPS and various inflammatory factors can inhibit osteoblast activity, promote osteoclast activation and proliferation, and break the periodontal steady state, all of which can indirectly or directly lead to alveolar bone absorption [
44]. Therefore, LPS stimulation of RAW264.7 cells with Porphyromonas gingivalis was used to establish the inflammation model in vitro, with high glucose stimulation given simultaneously (50 mmol glucose per elevated glucose medium); 5.5 mmol/L low-glucose medium was used as the base medium, and 406 mg glucose powder was dissolved in 30 mL DMEM until fully dissolved. Next, the solution was filter sterilized with a 0.22 μm filter before adding DMEM, FBS, and double antibody to a constant volume of 50 mL. To evaluate the anti-inflammatory effect of IL-1ra CS/β-GP/Gel temperature-sensitive hydrogels, we measured the mRNA expression of inflammatory cytokines in RAW264.7 cells under high glucose conditions in vitro by quantitative real-time polymerase chain reaction. RAW264.7 cells were cultured under the conditions outlined in
Section 2.5. The specific groups were as follows: (1) Blank control group: RAW264.7 without any treatment; (2) LPS stimulation group: RAW264.7 cells were stimulated with LPS (5 μg/mL) for 24 h; (3) LPS + high glucose stimulation group: RAW264.7 cells were stimulated with LPS (5 μg/mL) in high glucose medium (containing 50 mmol/L glucose) for 24 h; (4) LPS + high glucose stimulation + blank hydrogel group: high glucose medium (containing 50 mmol/L glucose) and blank hydrogel (CS/β-GP/Gel temperature-sensitive hydrogel without IL-1ra) were co-cultured for 24 h. The collected high glucose gel extract was used to stimulate RAW264.7 cells for 3 h, while LPS (5 μg/mL) was used to stimulate RAW264.7 cells for 24 h; and (5) LPS + high glucose stimulation + IL-1ra CS/β-GP/Gel thermosensitive hydrogel group: CS/β-GP/Gel temperature-sensitive hydrogel loaded with IL-1ra was cultured in high glucose medium (containing 50 mmoL/L glucose) for 24 h. The collected high glucose hydrogel extracts were used to stimulate RAW264.7 cells for 3 h, and LPS (5 μg/mL) was used to stimulate RAW264.7 cells for 4 h. According to Lu’s method [
39], RT-qPCR was used to detect the mRNA expression of IL-1β, IL-6, and TNF-α in each group. The primer sequences used in RT-qPCR are shown in
Table 3.
4.5. In Vivo Toxicity of IL-1ra CS/β-GP/Gel Thermosensitive Hydrogel
We used male Wistar rats, 6–8 weeks old, average weight 240–260 g. Thirty-six male Wistar rats were randomly divided into six groups: blank group (group I), periodontitis group (group II), diabetic periodontitis group (group III), diabetic periodontitis + blank hydrogel treatment group (group IV), diabetic periodontitis + IL-1ra alone group (group V), and diabetic periodontitis + IL-1ra CS/β-GP/Gel thermosensitive hydrogel treatment group (group VI). The rat model of diabetes was established by intraperitoneal injection of streptozotocin (STZ) 60 mg/kg. On the 3rd and 7th day after injection, blood was collected from the tail vein to detect the blood glucose level of rats; a blood glucose level > 16.7 mmol/L recorded twice indicated that the modeling was successful. The model of diabetes mellitus was successfully established, and the rat periodontitis model was established simultaneously; that is, the rats were anesthetized by intraperitoneal injection of 1% pentobarbital sodium. A 0.2 mm orthodontic ligature wire was placed between the first molar and the second molar of the maxilla of the rats. On the day when the periodontitis model was established, 50 μL of thermosensitive hydrogel containing 50 μg/mL IL-1ra was injected into the buccal-palatal gingival mucosa of the maxillary first molar of the rat model side. Inject once a week until the end of the experiment, a total of four times. Rats were anesthetized by intraperitoneal injection of 1% pentobarbital sodium after 4 weeks, and then euthanized. The bilateral maxilla, periodontal tissues, and liver and kidney tissue of rats were taken for subsequent experiments.
Four weeks after modeling, the rats were anesthetized with 1% pentobarbital sodium intraperitoneally. Next, 3 mL blood was collected from the right ventricle and supernatant was taken by a static method to detect the levels of serum creatinine (Cr), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). After fixation for 48 h, the liver and kidney tissue samples were rinsed with water to remove the tissue fixative fluid, dehydrated and embedded, and then sectioned for hematoxylin and eosin (H&E) staining for histological analysis.
4.6. Effect of IL-1ra-Loaded CS/β-GP/Gelatin Thermosensitive Hydrogel on Body Weight and Blood Glucose in Rats and Its Anti-Inflammatory Properties In Vivo
The weight of rats was recorded from the first day of ligation. Three rats in each group were randomly selected and weighed at 12 noon every day, and the average value was taken for two weeks. After the establishment of the diabetes model, tail venous blood of rats in each group was collected at the same time every week, and the blood glucose level was monitored by a glucose meter at the end of the experiment. The collected serum was thawed again, and the serum glycosylated hemoglobin (HbA1c) level of rats in each group was determined by ELISA (ELISA; Meimian, Suzhou, Jiangsu, China).
The total RNA of rat maxillary alveolar bone preserved in liquid nitrogen was extracted by TRIzol (Life Technologies, Carlsbad, CA, USA), and then reverse-transcribed and amplified by qRT-PCR (Takara, Kusatsu, Shiga, Japan). The grouping was as outlined in
Section 4.5, and the statistical analysis method was the same as that outlined in
Section 4.4.
4.7. Micro-CT
The alveolar bone of rats was fixed with 4% paraformaldehyde for 48 h, and then fixed in a scanning container for CT scanning (SkyScan 1172; Bruker, Germany, 24 kV, 2 mA, 90 s). The level of alveolar bone between the first and the second molars was analyzed in the sagittal plane. The three-dimensional images were reconstructed by IPL image processing software (Scanco, Bassersdorf, Zurich, Switzerland). The distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) of each sample was taken as an ABL value (ABL = CEJ − ABC). For each tooth, we recorded six points in the buccal mesial, central and distal, and palatal mesial, central, and distal. The alveolar bone between the first and second molars was analyzed using bone parameter analysis software, including the bone volume fraction (BV/TV) and trabecular thickness (Tb.Th).
4.8. Histological Evaluation of Periodontitis in Diabetic Rats with IL-1ra CS/β-GP/Gel Thermosensitive Hydrogel
The Micro-CT treated samples were submerged in 10% EDTA for decalcification for 3 months, and the decalcified solution was changed every 2 days. After decalcification, the tissues were dehydrated, embedded, and sliced. The slicing was performed along the long axis of the first premolars, and the slices were evenly cut from the middle to the center and the far middle, with a thickness of approximately 3 μm. H&E staining was performed to evaluate the inflammation of rat periodontal tissue. IL-1β and IL-6 antibodies (Sangon, Shanghai, China) were used to perform immunohistochemical staining (IHC) on the tissue sections, and the staining of the upper and the first molar and the second molar (the ligation site) was observed with an optical microscope (BX51, Olympus, Tokyo, Japan). At least five fields of view with a magnification of ×400 were randomly selected, and the immune response score (IRS) of the area was calculated. The presence of a brownish yellow precipitate was regarded as an immunohistochemical positive reaction, and the cell staining intensity (SI) was divided into four grades as follows: no positive staining or negative score, 0 point; light yellow or weak positive score, 1 point; brown or positive score, 2 points; and brown or strong positive score, 3 points. The percentage of positive cells (PP) was divided into four grades as follows: the number of positive cells accounts for ≤25% of the total number of cells, score 1 point; 26–50%, 2 points; 51–75%, 3 points; and >75%, 4 points. The IRS was calculated as follows: IRS = SI × PP.
4.9. Statistical Analysis
All of the in vivo and in vitro experiments were performed in triplicate and repeated thrice. The experimental data were statistically analyzed using SPSS v23.0 software and plotted with Prism GraphPad v8.0 software (GraphPad by Dotmatics, San Diego, CA, USA). One-way ANOVA was used to compare the difference among three groups, and all calculated data are expressed as ± s. p-values < 0.05 were considered to be statistically significant.