Hyperpolarization Induced by Lipopolysaccharides but Not by Chloroform Is Inhibited by Doxapram, an Inhibitor of Two-P-Domain K+ Channel (K2P)
Abstract
:1. Introduction
2. Results
2.1. Effects of Varying Concentration of Chloroform on Muscle Cell Membrane Potential
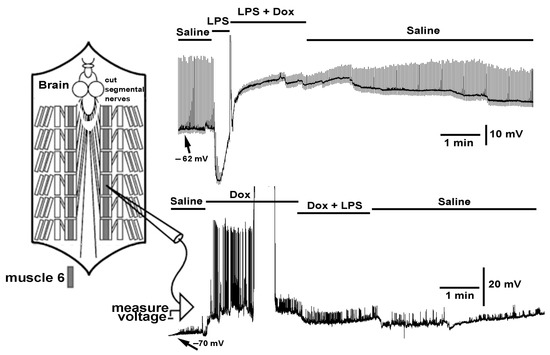
2.2. Effect LPS on the Resting Membrane Potential and LPS Combined with Chloroform
2.3. Effect of Electrically Stimulated Nerve While Exposed to Various Preparations
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Neuromuscular Junctions of Larval Drosophila
4.3. Chemicals
4.4. Statistical Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osborn, M.J.; Rosen, S.M.; Rothfield, L.; Zeleznick, L.D.; Horecker, B.L. Lipopolysaccharide of the gram-negative cell wall. Science 1964, 145, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Linhartová, I.; Bumba, L.; Mašín, J.; Basler, M.; Osička, R.; Kamanová, J.; Prochazkova, K.; Adkins, I.; Holubova, J.; Sadilkova, L. RTX proteins: A highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 2010, 34, 1076–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Correia, J.; Soldau, K.; Christen, U.; Tobias, P.S.; Ulevitch, R.J. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 2001, 276, 21129–21135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.X.; Young, G.B. Progress in clinical neurosciences: Sepsis-associated encephalopathy: Evolving concepts. Can. J. Neurol. Sci. 2003, 30, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, O.; Reid, M.B.; Van den Berghe, G.; Vanhorebeek, I.; Hermans, G.; Rich, M.M.; Larsson, L. The sick and the weak: Neuropathies/myopathies in the critically ill. Physiol. Rev. 2015, 95, 1025–1109. [Google Scholar] [CrossRef] [Green Version]
- Al-Nassan, S.; Fujino, H. Exercise preconditioning attenuates atrophic mediators and preserves muscle mass in acute sepsis. Gen. Physiol. Biophys. 2018, 37, 433–441. [Google Scholar] [CrossRef]
- Eidelman, L.A.; Putterman, D.; Putterman, C.; Sprung, C.L. The spectrum of septic encephalopathy definitions, etiologies, and mortalities. JAMA 1996, 275, 470–473. [Google Scholar] [CrossRef]
- Levin, T.C.; Malik, H.S. Rapidly evolving Toll-3/4 genes encode male-specific Toll-like receptors in Drosophila. Mol. Biol. Evol. 2017, 34, 2307–2323. [Google Scholar] [CrossRef] [Green Version]
- Coscia, M.; Giacomelli, S.; Oreste, U. Toll-like receptors: An overview from invertebrates to vertebrates. Invert. Surv. J. 2011, 8, 210–226. [Google Scholar]
- Loker, E.S.; Adema, C.M.; Zhang, S.M.; Kepler, T.B. Invertebrate immune systems—Not homogeneous, not simple, not well understood. Immunol. Rev. 2004, 198, 10–24. [Google Scholar] [CrossRef]
- Kleino, A.; Silverman, N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Com. Immunol. 2014, 42, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Gottar, M.; Gobert, V.; Michel, T.; Belvin, M.; Duyk, G.; Hoffmann, J.A.; Ferrandon, D.; Royet, J. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 2002, 416, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Takehana, A.; Katsuyama, T.; Yano, T.; Oshima, Y.; Takada, H.; Aigaki, T.; Kurata, S. Overexpression of a pattern-recognition receptor, peptidoglycan recognition protein-LE, activates imd/relish mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larvae. Proc. Natl. Acad. Sci. USA 2002, 99, 13705–13710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclerc, V.; Reichhart, J.M. The immune response of Drosophila melanogaster. Immunol. Rev. 2004, 198, 59–71. [Google Scholar] [CrossRef]
- Werner, T.; Liu, G.; Kang, D.; Ekengren, S.; Steiner, H.; Hultmark, D. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 13772–13777. [Google Scholar] [CrossRef] [Green Version]
- Perkins, L.A.; Holderbaum, L.; Tao, R.; Hu, Y.; Sopko, R.; McCall, K.; Yang-Zhou, D.; Flockhart, I.; Binari, R.; Shim, H.-S.; et al. The transgenic RNAi project at Harvard Medical School: Resources and validation. Genetics 2015, 201, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Ballinger-Boone, C.; Anyagaligbo, O.; Bernard, J.; Bierbower, S.M.; Dupont-Versteegden, E.E.; Ghoweri, A.; Greenhalgh, A.; Harrison, D.; Istas, O.; McNabb, M.; et al. The effects of bacterial endotoxin (LPS) on cardiac and synaptic function in various animal models: Larval Drosophila, crayfish, crab, and rodent. Internat. J. Zool. Res. 2020, 16, 33–62. [Google Scholar] [CrossRef]
- Vacassenno, R.M.; Haddad, C.N.; Cooper, R.L. Lipopolysaccharide (LPS) action on hyperpolarizing membrane potential: Antagonized by the K2P channel blocker, Doxapram, and independent of calcium activated potassium channels. 2022; in review. [Google Scholar]
- Ueda, I.; Hirakawa, M.; Arakawa, K.; Kamaya, H. Do anesthetics fluidize membranes? Anesthesiology 1986, 64, 67–71. [Google Scholar] [CrossRef]
- Hao, X.; Ou, M.; Zhang, D.; Zhao, W.; Yang, Y.; Liu, J.; Yang, H.; Zhu, T.; Li, Y.; Zhou, C. The effects of general anesthetics on synaptic transmission. Curr. Neuropharmacol. 2020, 18, 936–965. [Google Scholar] [CrossRef]
- Buckingham, S.D.; Kidd, J.F.; Law, R.J.; Franks, C.J.; Sattelle, D.B. Structure and function of two-pore-domain K+ channels: Contributions from genetic model organisms. Trends Pharmacol. Sci. 2005, 26, 361–367. [Google Scholar] [CrossRef]
- Plant, L.D.; Goldstein, S.A.N. Two-Pore Domain Potassium Channels. In Handbook of Ion Channels, 1st ed.; Zheng, J., Trudeau, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9780429193965. [Google Scholar]
- Goldstein, S.A.; Price, L.A.; Rosenthal, D.N.; Pausch, M.H. ORK1, a potassium-selective leak channel with two pore domains cloned from Drosophila melanogaster by expression in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1996, 93, 13256–13261. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A.; Wang, K.W.; Ilan, N.; Pausch, M.H. Sequence and function of the two P domain potassium channels: Implications of an emerging superfamily. J. Mol. Med. 1998, 76, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.M.; Müntefering, T.; Budde, T.; Meuth, S.G.; Ruck, T. Pathophysiological role of K2P channels in human diseases. Cell Physiol. Biochem. 2021, 55, 65–86. [Google Scholar] [PubMed]
- Schmidt, C.; Wiedmann, F.; Schweizer, P.A.; Katus, H.A.; Thomas, D. Inhibition of cardiac two-pore-domain K+ (K2P) channels--an emerging antiarrhythmic concept. Eur. J. Pharmacol. 2014, 738, 250–255. [Google Scholar] [CrossRef]
- Cotten, J.F.; Keshavaprasad, B.; Laster, M.J.; Eger, E.I., II; Yost, C.S. The ventilatory stimulant doxapram inhibits TASK tandem pore (K2P) potassium channel function but does not affect minimum alveolar anesthetic concentration. Anesth. Analg. 2006, 102, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, R.; Sengupta, P.; Cherynak, G.; Wadhwa, A.; Sessler, D.I.; Liu, J.; Hurst, H.E.; Lenhardt, R. Doxapram only slightly reduces the shivering threshold in healthy volunteers. Anesth. Analg. 2005, 101, 1368–1373. [Google Scholar] [CrossRef] [Green Version]
- Yost, C.S. A new look at the respiratory stimulant doxapram. CNS Drug Rev. Fall-Winter 2006, 12, 236–249. [Google Scholar] [CrossRef]
- Song, S.S.; Lyden, P.D. Overview of therapeutic hypothermia. Curr. Treat. Options. Neurol. 2012, 14, 541–548. [Google Scholar] [CrossRef]
- Cunningham, K.P.; MacIntyre, D.E.; Mathie, A.; Veale, E.L. Effects of the ventilatory stimulant, doxapram on human TASK-3 (KCNK9, K2P9.1) channels and TASK-1 (KCNK3, K2P3.1) channels. Acta Physiol. 2020, 228, e13361. [Google Scholar] [CrossRef]
- Vliegenthart, R.J.; Ten Hove, C.H.; Onland, W.; van Kaam, A.H. Doxapram treatment for apnea of prematurity: A systematic review. Neonatology 2017, 111, 162–171. [Google Scholar] [CrossRef]
- Sauter, C.; Wolfensberger, C. Interferon in human serum after injection of endotoxin. Lancet 1980, 316, 852–853. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.; Hoskins, R.A.; Galle, R.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Littleton, J.T.; Ganetzky, B. Ion channels and synaptic organization: Analysis of the Drosophila genome. Neuron 2000, 26, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Enyedi, P.; Braun, G.; Czirják, G. TRESK: The lone ranger of two-pore domain potassium channels. Mol. Cell Endocrinol. 2012, 353, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kamuene, J.M.; Xu, Y.; Plant, L.D. The pharmacology of two-pore domain potassium channels. Handb. Exp. Pharmacol. 2021, 267, 417–443. [Google Scholar]
- Duprat, F.; Lesage, F.; Fink, M.; Reyes, R.; Heurteaux, C.; Lazdunski, M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997, 16, 5464–5471. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Bang, H.; Kim, D. TASK-3, a new member of the tandem pore K(+) channel family. J. Biol. Chem. 2000, 275, 9340–9347. [Google Scholar] [CrossRef] [Green Version]
- Rajan, S.; Wischmeyer, E.; Xin Liu, G.; Preisig-Muller, R.; Daut, J.; Karschin, A.; Derst, C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histidine as pH sensor. J. Biol. Chem. 2000, 275, 16650–16657. [Google Scholar] [CrossRef] [Green Version]
- Kim, D. Physiology and pharmacology of two-pore domain potassium channels. Curr. Pharm. Des. 2005, 11, 2717–2736. [Google Scholar] [CrossRef]
- Mu, D.; Chen, L.; Zhang, X.; See, L.-H.; Koch, C.M.; Yen, C.; Tong, J.J.; Spiegel, L.; Nguyen, K.C.; Servoss, A.; et al. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell 2003, 3, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Holter, J.; Carter, D.; Leresche, N.; Crunelli, V.; Vincent, P. A TASK3 channel (KCNK9) mutation in a genetic model of absence epilepsy. J. Mol. Neurosci. 2005, 25, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Honore, E.; Lesage, F.; Fink, M.; Romey, G.; Lazdunski, M. Inhalational anesthetics activate two-pore-domain background K channels. Nat. Neurosci. 1999, 2, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Qiu, Y.; Lan, X.; Li, M.; Yang, H.; Gao, Z. A small-molecule compound selectively activates K2P channel TASK-3 by acting at two distant clusters of residues. Mol. Pharmacol. 2019, 96, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Badre, N.H.; Martin, M.E.; Cooper, R.L. The physiological and behavioral effects of carbon dioxide on Drosophila melanogaster larvae. Comp. Biochem. Physiol. A. 2005, 140, 363–376. [Google Scholar] [CrossRef]
- Vacassenno, R.M.; Haddad, C.N.; Cooper, R.L. The effects on resting membrane potential and synaptic transmission by Doxapram (blocker of K2P channels) at the Drosophila neuromuscular junction. Comp. Biochem. Physiol. C 2023, 263, 109497. [Google Scholar] [CrossRef]
- Kollert, S.; Döring, F.; Gergs, U.; Wischmeyer, E. Chloroform is a potent activator of cardiac and neuronal Kir3 channels. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 573–580. [Google Scholar] [CrossRef]
- Andres-Enguix, I.; Caley, A.; Yustos, R.; Schumacher, M.A.; Spanu, P.D.; Dickinson, R.; Maze, M.; Franks, N.P. Determinants of the anesthetic sensitivity of two-pore domain acid-sensitive potassium channels: Molecular cloning of an anesthetic-activated potassium channel from Lymnaea stagnalis. J. Biol. Chem. 2007, 282, 20977–20990. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Yoshida, H. Drosophila as a model organism. Adv. Exp. Med. Biol. 2018, 1076, 1–10. [Google Scholar]
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila tools and assays for the study of human diseases. Dis. Model. Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Ecovoiu, A.A.; Ratiu, A.C.; Micheu, M.M.; Chifiriuc, M.C. Inter-Species Rescue of Mutant Phenotype-The Standard for Genetic Analysis of Human Genetic Disorders in Drosophila melanogaster Model. Int. J. Mol. Sci. 2022, 23, 2613. [Google Scholar] [CrossRef]
- Dow, J.A.T.; Simons, M.; Romero, M.F. Drosophila melanogaster: A simple genetic model of kidney structure, function and disease. Nat. Rev. Nephrol. 2022, 18, 417–434. [Google Scholar] [CrossRef]
- Shin, G.J.; Abaci, H.E.; Smith, M.C. Cellular pathogenesis of chemotherapy-induced peripheral neuropathy: Insights from Drosophila and Human-engineered skin models. Front. Pain Res. 2022, 3, 912977. [Google Scholar] [CrossRef] [PubMed]
- Mackay, T.F.; Anholt, R.R. Of flies and man: Drosophila as a model for human complex traits. Annu. Rev. Genom. Hum. Genet. 2006, 7, 339–367. [Google Scholar] [CrossRef]
- Cooper, R.L.; McNabb, M.; Nadolski, J. The effects of a bacterial endotoxin LPS on synaptic transmission at the neuromuscular junction. Heliyon 2019, 5, e01430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donohoe, P.B.; Huskens, N.; Turner, P.J.; Pandit, J.J.; Buckler, K.J. A1899, PK-THPP, ML365, and Doxapram inhibit endogenous TASK channels and excite calcium signaling in carotid body type-1 cells. Physiol. Rep. 2018, 6, e13876. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Ozawa, S.; Hagiwara, S. Synaptic transmission reversibly conditioned by single-gene mutation in Drosophila melanogaster. Nature 1976, 259, 489–491. [Google Scholar] [CrossRef]
- Salkoff, L.B.; Wyman, R.J. Ion currents in Drosophila flight muscles. J. Physiol. 1983, 337, 687–709. [Google Scholar] [CrossRef]
- Potter, R.; Meade, A.; Potter, S.; Cooper, R.L. Rapid and direct action of lipopolysaccharides (LPS) on skeletal muscle of larval Drosophila. Biology 2021, 10, 1235. [Google Scholar] [CrossRef]
- Bierbower, S.M.; Cooper, R.L. The effects of acute carbon dioxide on behavior and physiology in Procambarus clarkii. J. Exp. Zool. 2010, 313A, 484–497. [Google Scholar] [CrossRef]
- Bierbower, S.M.; Shuranova, Z.P.; Viele, K.; Cooper, R.L. Comparative study of environmental factors influencing motor task learning and memory retention in sighted and blind crayfish. Brain Behav. 2013, 3, 4–13. [Google Scholar] [CrossRef]
- Anyagaligbo, O.; Bernard, J.; Greenhalgh, A.; Cooper, R.L. The effects of bacterial endotoxin (LPS) on cardiac function in a medicinal blow fly (Phaenicia sericata) and a fruit fly (Drosophila melanogaster). Comp. Biochem. Physiol. C 2019, 217, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Istas, O.; Greenhalgh, A.; Cooper, R.L. The effects of a bacterial endotoxin on behavior and sensory-CNS-motor circuits in Drosophila melanogaster. Insects 2019, 10, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Istas, O.; Greenhalgh, A.; Cooper, R.L. Repetitive exposure to bacterial endotoxin LPS alters synaptic transmission. J. Pharmacol. Toxicol. 2020, 15, 65–72. [Google Scholar] [CrossRef]
- Saelinger, C.M.; McNabb, M.C.; McNair, R.; Bierbower, S.; Cooper, R.L. Effects of bacterial endotoxin (LPS) on the cardiac function, neuromuscular transmission and sensory-CNS-motor nerve circuit: A crustacean model. Comp. Biochem. Physiol. A 2019, 237, 110557. [Google Scholar] [CrossRef] [PubMed]
- Keshavaprasad, B.; Liu, C.; Au, J.D.; Kindler, C.H.; Cotten, J.F.; Yost, C.S. Species-specific differences in response to anesthetics and other modulators by the K2P channel TRESK. Anesth. Analg. 2005, 101, 1042–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Kim, E.-J.; Ryu, J.H.; Lee, D.K.; Hong, S.-G.; Han, J.; Han, J.; Kang, D. Verapamil inhibits TRESK (K2P18.1) current in trigeminal ganglion neurons independently of the blockade of Ca2+ influx. Int. J. Mol. Sci. 2018, 19, 1961. [Google Scholar] [CrossRef] [Green Version]
- Cotton, J.F. TASK-1 (KCNK3) and TASK-3 (KCNK9) tandem pore potassium channel antagonists stimulate breathing in isoflurane-anesthetized rats. Anesth. Analg. 2013, 116, 810–816. [Google Scholar] [CrossRef] [Green Version]
- Ison, B.J.; Abul-Khoudoud, M.O.; Ahmed, S.; Alhamdani, A.W.; Ashley, C.; Bidros, P.C.; Bledsoe, C.O.; Bolton, K.E.; Capili, J.G.; Henning, J.N.; et al. The effect of doxapram on proprioceptive neurons: Invertebrate model. NeuroSci 2022, 3, 566–588. [Google Scholar] [CrossRef]
- Stewart, B.A.; Atwood, H.L.; Renger, J.J.; Wang, J.; Wu, C.F. Improved stability of Drosophila larval neuromuscular preparation in haemolymph-like physiological solutions. J. Comp. Physiol. A 1994, 175, 179–191. [Google Scholar] [CrossRef]
- De Castro, C.; Titlow, J.; Majeed, Z.R.; Cooper, R.L. Analysis of various physiological salines for heart rate, CNS function, and synaptic transmission at neuromuscular junctions in Drosophila melanogaster larvae. J. Comp. Physiol. A 2014, 200, 83–92. [Google Scholar] [CrossRef]
- Iwaya, A.; Nakagawa, S.; Iwakura, N.; Taneike, I.; Kurihara, M.; Kuwano, T.; Gondaira, F.; Endo, M.; Hatakeyama, K.; Yamamoto, T. Rapid and quantitative detection of blood Serratia marcescens by a real-time PCR assay: Its clinical application and evaluation in a mouse infection model. FEMS Microbiol. Lett. 2005, 248, 163–170. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, R.L.; Krall, R.M. Hyperpolarization Induced by Lipopolysaccharides but Not by Chloroform Is Inhibited by Doxapram, an Inhibitor of Two-P-Domain K+ Channel (K2P). Int. J. Mol. Sci. 2022, 23, 15787. https://doi.org/10.3390/ijms232415787
Cooper RL, Krall RM. Hyperpolarization Induced by Lipopolysaccharides but Not by Chloroform Is Inhibited by Doxapram, an Inhibitor of Two-P-Domain K+ Channel (K2P). International Journal of Molecular Sciences. 2022; 23(24):15787. https://doi.org/10.3390/ijms232415787
Chicago/Turabian StyleCooper, Robin L., and Rebecca M. Krall. 2022. "Hyperpolarization Induced by Lipopolysaccharides but Not by Chloroform Is Inhibited by Doxapram, an Inhibitor of Two-P-Domain K+ Channel (K2P)" International Journal of Molecular Sciences 23, no. 24: 15787. https://doi.org/10.3390/ijms232415787
APA StyleCooper, R. L., & Krall, R. M. (2022). Hyperpolarization Induced by Lipopolysaccharides but Not by Chloroform Is Inhibited by Doxapram, an Inhibitor of Two-P-Domain K+ Channel (K2P). International Journal of Molecular Sciences, 23(24), 15787. https://doi.org/10.3390/ijms232415787