Imaging the Kidney with an Unconventional Scanning Electron Microscopy Technique: Analysis of the Subpodocyte Space in Diabetic Mice
Abstract
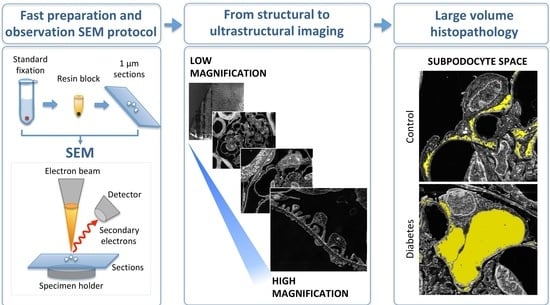
:1. Introduction
2. Results
2.1. TEM-like Images from Resin-Embedded Semi-Thin Sections Using SE–SEM
2.2. Characterisation of Kidney Functional Parameters of BTBR ob/ob Mice
2.3. SE–SEM Ultrastructural Analysis in BTBR ob/ob Mice
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Biochemical Parameters
4.3. Tissue Fixation and Processing for SE–SEM Ultrastructural Analysis
4.4. Sections Preparation for SE–SEM Ultrastructural Analysis
4.5. SE–SEM Imaging Acquisition
4.6. Ultrastructure Morphometrical Analysis on SE–SEM Images
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knoll, M.; Ruska, E. Das Elektronenmikroskop. Z. Phys. 1932, 78, 318–339. [Google Scholar] [CrossRef]
- Kruger, D.H.; Schneck, P.; Gelderblom, H.R. Helmut Ruska and the visualisation of viruses. Lancet Lond. Engl. 2000, 355, 1713–1717. [Google Scholar] [CrossRef]
- Herrera, G.A.; Isaac, J.; Turbat-Herrera, E.A. Role of Electron Microscopy in Transplant Renal Pathology. Ultrastruct. Pathol. 1997, 21, 481–498. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.A. The Continuing Value of Electron Microscopy in Surgical Pathology. Ultrastruct. Pathol. 2000, 24, 383–389. [Google Scholar] [CrossRef]
- Ivanyi, B.; Kemeny, E.; Szederkenyi, E.; Marofka, F.; Szenohradszky, P. The Value of Electron Microscopy in the Diagnosis of Chronic Renal Allograft Rejection. Mod. Pathol. 2001, 14, 1200–1208. [Google Scholar] [CrossRef]
- Smith, D.J. Ultimate resolution in the electron microscope? Mater. Today 2008, 11, 30–38. [Google Scholar] [CrossRef]
- Carrara, C.; Abbate, M.; Conti, S.; Rottoli, D.; Rizzo, P.; Marchetti, G. Histological Examination of the Diabetic Kidney. Methods Mol. Biol. 2020, 2067, 63–87. [Google Scholar]
- Ul-Hamid, A. Introduction. In A Beginners’ Guide to Scanning Electron Microscopy; Ul-Hamid, A., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–14. [Google Scholar]
- Conti, S.; Perico, N.; Novelli, R.; Carrara, C.; Benigni, A.; Remuzzi, G. Early and late scanning electron microscopy findings in diabetic kidney disease. Sci. Rep. 2018, 8, 4909. [Google Scholar] [CrossRef]
- Reichelt, M.; Sagolla, M.; Katakam, A.K.; Webster, J.D. Unobstructed Multiscale Imaging of Tissue Sections for Ultrastructural Pathology Analysis by Backscattered Electron Scanning Microscopy. J. Histochem. Cytochem. 2019, 68, 9–23. [Google Scholar] [CrossRef]
- Masum, M.A.; Ichii, O.; Elewa, Y.H.A.; Nakamura, T.; Otani, Y.; Hosotani, M.; Kon, Y. Modified scanning electron microscopy reveals pathological crosstalk between endothelial cells and podocytes in a murine model of membranoproliferative glomerulonephritis. Sci. Rep. 2018, 8, 10276. [Google Scholar] [CrossRef]
- Koga, D.; Kusumi, S.; Shodo, R.; Dan, Y.; Ushiki, T. High-resolution imaging by scanning electron microscopy of semithin sections in correlation with light microscopy. Microscopy 2015, 64, 387–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alpers, C.E.; Hudkins, K.L. Mouse models of diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 2011, 20, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, A.; Conti, S.; Ene-Iordache, B.; Tomasoni, S.; Rizzo, P.; Benigni, A.; Remuzzi, G. Role of ultrastructural determinants of glomerular permeability in ultrafiltration function loss. JCI Insight 2020, 5, e137249. [Google Scholar] [CrossRef] [PubMed]
- Evekhart, T.E.; Wells, O.O.; Oatley, C.W. Factors Affecting Contrast and Resolution in the Scanning Electron Microscope. J. Electron. Control 1959, 7, 97–111. [Google Scholar] [CrossRef]
- Roussel, L.Y.; Stokes, D.J.; Gestmann, I.; Darus, M.; Young, R.J. Extreme high resolution scanning electron microscopy (XHR SEM) and beyond. Scanning Microscopy 2009. Int. Soc. Opt. Photonics 2009, 7378, 73780W. [Google Scholar]
- Mitchell, D.R.G.; Casillas, G. Secondary Electron Imaging in an Aberration-Corrected STEM. Microsc. Today 2016, 24, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Inada, H.; Su, D.; Egerton, R.F.; Konno, M.; Wu, L.; Ciston, J.; Wall, J.; Zhu, Y. Atomic imaging using secondary electrons in a scanning transmission electron microscope: Experimental observations and possible mechanisms. Ultramicroscopy 2011, 111, 865–876. [Google Scholar] [CrossRef] [Green Version]
- Hudkins, K.L.; Pichaiwong, W.; Wietecha, T.; Kowalewska, J.; Banas, M.C.; Spencer, M.W.; Mühlfeld, A.; Koelling, M.; Pippin, J.W.; Shankland, S.J.; et al. BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 1533–1542. [Google Scholar] [CrossRef] [Green Version]
- Pichaiwong, W.; Hudkins, K.L.; Wietecha, T.; Nguyen, T.Q.; Tachaudomdach, C.; Li, W.; Askari, B.; Kobayashi, T.; O’Brien, K.; Pippin, J.W.; et al. Reversibility of Structural and Functional Damage in a Model of Advanced Diabetic Nephropathy. J. Am. Soc. Nephrol. 2013, 24, 1088–1102. [Google Scholar] [CrossRef] [Green Version]
- Attie, A.D.; Schueler, K.M.; Keller, M.P.; Mitok, K.A.; Simonett, S.P.; Hudkins, K.L.; Mehrotra, K.; Graham, M.J.; Lee, R.G.; Alpers, C.E. Reversal of hypertriglyceridemia in diabetic BTBR ob/ob mice does not prevent nephropathy. Lab. Investig. 2021, 101, 935–941. [Google Scholar] [CrossRef]
- Gembardt, F.; Bartaun, C.; Jarzebska, N.; Mayoux, E.; Todorov, V.T.; Hohenstein, B.; Hugo, C. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am. J. Physiol. Ren. Physiol. 2014, 307, F317–F325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassis, P.; Locatelli, M.; Corna, D.; Villa, S.; Rottoli, D.; Cerullo, D.; Abbate, M.; Remuzzi, G.; Benigni, A.; Zoja, C. Addition of cyclic angiotensin-(1-7) to angiotensin-converting enzyme inhibitor therapy has a positive add-on effect in experimental diabetic nephropathy. Kidney Int. 2019, 96, 906–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudkins, K.L.; Wietecha, T.A.; Steegh, F.; Alpers, C.E. Beneficial effect on podocyte number in experimental diabetic nephropathy resulting from combined atrasentan and RAAS inhibition therapy. Am. J. Physiol. Renal Physiol. 2020, 318, F1295–F1305. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Zoja, C.; Zanchi, C.; Corna, D.; Villa, S.; Bolognini, S.; Novelli, R.; Perico, L.; Remuzzi, G.; Benigni, A.; et al. Manipulating Sirtuin 3 pathway ameliorates renal damage in experimental diabetes. Sci. Rep. 2020, 10, 8418. [Google Scholar] [CrossRef]
- Lee, S.H.; Moon, S.J.; Paeng, J.; Kang, H.-Y.; Nam, B.Y.; Kim, S.; Kim, C.H.; Lee, M.J.; Oh, H.J.; Park, J.T.; et al. Podocyte hypertrophy precedes apoptosis under experimental diabetic conditions. Apoptosis 2015, 20, 1056–1071. [Google Scholar] [CrossRef]
- Pearson, J.M.; McWilliam, L.J.; Coyne, J.D.; Curry, A. Value of electron microscopy in diagnosis of renal disease. J. Clin. Pathol. 1994, 47, 126–128. [Google Scholar] [CrossRef] [Green Version]
- Pavlisko, E.N.; Howell, D.N. The Continued Vital Role of Electron Microscopy in the Diagnosis of Renal Disease/Dysfunction. Ultrastruct. Pathol. 2013, 37, 1–8. [Google Scholar] [CrossRef]
- L’Imperio, V.; Brambilla, V.; Cazzaniga, G.; Ferrario, F.; Nebuloni, M.; Pagni, F. Digital pathology for the routine diagnosis of renal diseases: A standard model. J. Nephrol. 2020, 34, 681–688. [Google Scholar] [CrossRef]
- Haruhara, K.; Sasaki, T.; de Zoysa, N.; Okabayashi, Y.; Kanzaki, G.; Yamamoto, I.; Harper, I.S.; Puelles, V.G.; Shimizu, A.; Cullen-McEwen, L.A.; et al. Podometrics in Japanese Living Donor Kidneys: Associations with Nephron Number, Age, and Hypertension. J. Am. Soc. Nephrol. 2021, 32, 1187–1199. [Google Scholar] [CrossRef]
- Hodgin, J.B.; Bitzer, M.; Wickman, L.; Afshinnia, F.; Wang, S.Q.; O’Connor, C.; Yang, Y.; Meadowbrooke, C.; Chowdhury, M.; Kikuchi, M.; et al. Glomerular Aging and Focal Global Glomerulosclerosis: A Podometric Perspective. J. Am. Soc. Nephrol. 2015, 26, 3162–3178. [Google Scholar] [CrossRef]
- Christensen, E.I.; Kristoffersen, I.B.; Grann, B.; Thomsen, J.S.; Andreasen, A.; Nielsen, R. A well-developed endolysosomal system reflects protein reabsorption in segment 1 and 2 of rat proximal tubules. Kidney Int. 2020, 99, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kwak, S.; Jung, D.; Kim, J.; Yoo, T.-H.; Ryu, D.-R.; Han, S.H.; Choi, H.Y.; Lee, J.E.; Moon, S.; et al. Podocyte biology in diabetic nephropathy. Kidney Int. 2007, 72, S36–S42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.K.; Nam, B.Y.; Li, J.J.; Park, J.T.; Lee, S.H.; Kim, D.H.; Kim, J.Y.; Kang, H.Y.; Han, S.H.; Yoo, T.H.; et al. Translationally controlled tumour protein is associated with podocyte hypertrophy in a mouse model of type 1 diabetes. Diabetologia 2012, 55, 1205–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmon, A.H.J.; Toma, I.; Sipos, A.; Muston, P.R.; Harper, S.J.; Bates, D.O.; Neal, C.R.; Peti-Peterdi, J. Evidence for restriction of fluid and solute movement across the glomerular capillary wall by the subpodocyte space. Am. J. Physiol. Physiol. 2007, 293, F1777–F1786. [Google Scholar] [CrossRef] [Green Version]
- Nakakoshi, M.; Nishioka, H.; Katayama, E. New versatile staining reagents for biological transmission electron microscopy that substitute for uranyl acetate. J. Electron. Microsc. 2011, 60, 401–407. [Google Scholar] [CrossRef]
- Weibel, E.R. Stereological Methods Volume 1: Practical Methods for Biological Morphometry; Academic Press Inc.: London, UK, 1979; Volume 1, p. 415. ISBN 0-12-742201-3. [Google Scholar]







| Groups | Blood Glucose (mg/dL) | Diuresis (mL/24 h) | SBP (mmHg) | UAE (µg/24 h) | U Creat (mg/dL) |
|---|---|---|---|---|---|
| BTBR WT | 104.67 ± 12.99 | 1.10 ± 0.25 | 102.67 ± 6.17 | 15.86 ± 2.01 | 1.31 ± 0.39 |
| BTBR ob/ob + vehicle | 531.33 ± 35.18 ** | 21.33 ± 9.22 | 91.00 ± 5.51 | 2396 ± 1548 | 41.71 ± 3.45 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, S.; Remuzzi, G.; Benigni, A.; Tomasoni, S. Imaging the Kidney with an Unconventional Scanning Electron Microscopy Technique: Analysis of the Subpodocyte Space in Diabetic Mice. Int. J. Mol. Sci. 2022, 23, 1699. https://doi.org/10.3390/ijms23031699
Conti S, Remuzzi G, Benigni A, Tomasoni S. Imaging the Kidney with an Unconventional Scanning Electron Microscopy Technique: Analysis of the Subpodocyte Space in Diabetic Mice. International Journal of Molecular Sciences. 2022; 23(3):1699. https://doi.org/10.3390/ijms23031699
Chicago/Turabian StyleConti, Sara, Giuseppe Remuzzi, Ariela Benigni, and Susanna Tomasoni. 2022. "Imaging the Kidney with an Unconventional Scanning Electron Microscopy Technique: Analysis of the Subpodocyte Space in Diabetic Mice" International Journal of Molecular Sciences 23, no. 3: 1699. https://doi.org/10.3390/ijms23031699
APA StyleConti, S., Remuzzi, G., Benigni, A., & Tomasoni, S. (2022). Imaging the Kidney with an Unconventional Scanning Electron Microscopy Technique: Analysis of the Subpodocyte Space in Diabetic Mice. International Journal of Molecular Sciences, 23(3), 1699. https://doi.org/10.3390/ijms23031699