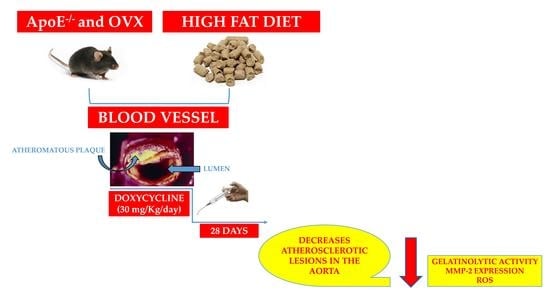
Doxycycline Decreases Atherosclerotic Lesions in the Aorta of ApoE-⁄- and Ovariectomized Mice with Correlation to Reduced MMP-2 Activity
Abstract
:1. Introduction
2. Results
2.1. Doxycycline Treatment Does Not Alter the Lipid Profile in ApoE-⁄-/OVX Mice
2.2. Doxycycline Reduced the Size of Atherosclerotic Lesions in ApoE-⁄-/OVX Animals
2.3. Doxycycline Decreases Gelatinolytic Activity and MMP-2 Expression in ApoE-⁄-/OVX In Situ Animals
2.4. Doxycycline Treatment Decreases ROS in ApoE-⁄-/OVX Animals
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Experimental Design
4.3. Obtaining Blood and Aorta Samples
4.4. Aortic Morphometry
4.5. Gelatinolytic Activity of MMP-2 in the Aorta by In Situ Zymography
4.6. MMP-2 Expression in the Aorta by Immunofluorescence
4.7. ROS Determination In Situ
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 30 November 2021).
- Aengevaeren, V.L.; Mosterd, A.; Sharma, S.; Prakken, N.H.J.; Mohlenkamp, S.; Thompson, P.D.; Velthuis, B.K.; Eijsvogels, T.M.H. Exercise and Coronary Atherosclerosis: Observations, Explanations, Relevance, and Clinical Management. Circulation 2020, 141, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Kereiakes, D.J.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W.; et al. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 76, 2635–2646. [Google Scholar] [CrossRef]
- Nandalur, K.R.; Baskurt, E.; Hagspiel, K.D.; Phillips, C.D.; Kramer, C.M. Calcified carotid atherosclerotic plaque is associated less with ischemic symptoms than is noncalcified plaque on MDCT. AJR Am. J. Roentgenol. 2005, 184, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Aboyans, V.; Criqui, M.H.; McClelland, R.L.; Allison, M.A.; McDermott, M.M.; Goff, D.C., Jr.; Manolio, T.A. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: The Multi-Ethnic Study of Atherosclerosis (MESA). J. Vasc. Surg. 2007, 45, 319–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef] [Green Version]
- Smabrekke, B.; Rinde, L.B.; Hindberg, K.; Hald, E.M.; Vik, A.; Wilsgaard, T.; Lochen, M.L.; Njolstad, I.; Mathiesen, E.B.; Hansen, J.B.; et al. Atherosclerotic Risk Factors and Risk of Myocardial Infarction and Venous Thromboembolism; Time-Fixed versus Time-Varying Analyses. The Tromso Study. PLoS ONE 2016, 11, e0163242. [Google Scholar] [CrossRef]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Krankel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Barrett-Connor, E. Menopause, atherosclerosis, and coronary artery disease. Curr. Opin. Pharmacol. 2013, 13, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.P.; Lee, W.S. Effects of female sex hormones on the development of atherosclerosis. Chin. J. Physiol. 2020, 63, 256–262. [Google Scholar] [CrossRef]
- Murano, T.; Izumi, S.; Kika, G.; Haque, S.F.; Okuwaki, S.; Mori, A.; Suzuki, T.; Matsubayashi, H.; Ikeda, M.; Goya, K.; et al. Impact of menopause on lipid and bone metabolism and effect of hormone replacement therapy. Tokai J. Exp. Clin. Med. 2003, 28, 109–119. [Google Scholar] [PubMed]
- Ferreira, C.N.; Carvalho, M.G.; Fernandes, A.P.; Lima, L.M.; Loures-Valle, A.A.; Dantas, J.; Janka, Z.; Palotas, A.; Sousa, M.O. Comparative study of apolipoprotein-E polymorphism and plasma lipid levels in dyslipidemic and asymptomatic subjects, and their implication in cardio/cerebro-vascular disorders. Neurochem. Int. 2010, 56, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Corbo, R.M.; Scacchi, R.; Mureddu, L.; Mulas, G.; Alfano, G. Apolipoprotein E polymorphism in Italy investigated in native plasma by a simple polyacrylamide gel isoelectric focusing technique. Comparison with frequency data of other European populations. Ann. Hum. Genet. 1995, 59, 197–209. [Google Scholar] [CrossRef]
- Emini Veseli, B.; Perrotta, P.; De Meyer, G.R.A.; Roth, L.; Van der Donckt, C.; Martinet, W.; De Meyer, G.R.Y. Animal models of atherosclerosis. Eur. J. Pharmacol. 2017, 816, 3–13. [Google Scholar] [CrossRef]
- Daniels, T.F.; Killinger, K.M.; Michal, J.J.; Wright, R.W., Jr.; Jiang, Z. Lipoproteins, cholesterol homeostasis and cardiac health. Int. J. Biol. Sci. 2009, 5, 474–488. [Google Scholar] [CrossRef]
- Huang, Z.H.; Minshall, R.D.; Mazzone, T. Mechanism for endogenously expressed ApoE modulation of adipocyte very low density lipoprotein metabolism: Role in endocytic and lipase-mediated metabolic pathways. J. Biol. Chem. 2009, 284, 31512–31522. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, D.; Wang, M.; Sun, J.Y.; Liu, J.; Qi, Y.; Hao, Y.C.; Deng, Q.J.; Liu, J.; Liu, J.; et al. Association of menopause with risk of carotid artery atherosclerosis. Maturitas 2021, 143, 171–177. [Google Scholar] [CrossRef]
- Borges, C.C.; Penna-de-Carvalho, A.; Medeiros Junior, J.L.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Ovariectomy modify local renin-angiotensin-aldosterone system gene expressions in the heart of ApoE (−/−) mice. Life Sci. 2017, 191, 1–8. [Google Scholar] [CrossRef]
- Laxton, R.C.; Hu, Y.; Duchene, J.; Zhang, F.; Zhang, Z.; Leung, K.Y.; Xiao, Q.; Scotland, R.S.; Hodgkinson, C.P.; Smith, K.; et al. A role of matrix metalloproteinase-8 in atherosclerosis. Circ. Res. 2009, 105, 921–929. [Google Scholar] [CrossRef]
- Galis, Z.S.; Johnson, C.; Godin, D.; Magid, R.; Shipley, J.M.; Senior, R.M.; Ivan, E. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ. Res. 2002, 91, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Kai, H.; Ikeda, H.; Yasukawa, H.; Kai, M.; Seki, Y.; Kuwahara, F.; Ueno, T.; Sugi, K.; Imaizumi, T. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 1998, 32, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Kuzuya, M.; Nakamura, K.; Sasaki, T.; Cheng, X.W.; Itohara, S.; Iguchi, A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1120–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalla, E.; Lamster, I.B.; Hofmann, M.A.; Bucciarelli, L.; Jerud, A.P.; Tucker, S.; Lu, Y.; Papapanou, P.N.; Schmidt, A.M. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Luttun, A.; Lutgens, E.; Manderveld, A.; Maris, K.; Collen, D.; Carmeliet, P.; Moons, L. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation 2004, 109, 1408–1414. [Google Scholar] [CrossRef] [Green Version]
- Soder, P.O.; Meurman, J.H.; Jogestrand, T.; Nowak, J.; Soder, B. Matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in blood as markers for early atherosclerosis in subjects with chronic periodontitis. J. Periodontal Res. 2009, 44, 452–458. [Google Scholar] [CrossRef]
- Wu, T.C.; Leu, H.B.; Lin, W.T.; Lin, C.P.; Lin, S.J.; Chen, J.W. Plasma matrix metalloproteinase-3 level is an independent prognostic factor in stable coronary artery disease. Eur. J. Clin. Investig. 2005, 35, 537–545. [Google Scholar] [CrossRef]
- Yamada, S.; Wang, K.Y.; Tanimoto, A.; Fan, J.; Shimajiri, S.; Kitajima, S.; Morimoto, M.; Tsutsui, M.; Watanabe, T.; Yasumoto, K.; et al. Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am. J. Pathol. 2008, 172, 1419–1429. [Google Scholar] [CrossRef] [Green Version]
- Prado, A.F.; Batista, R.I.M.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix Metalloproteinases and Arterial Hypertension: Role of Oxidative Stress and Nitric Oxide in Vascular Functional and Structural Alterations. Biomolecules 2021, 11, 585. [Google Scholar] [CrossRef]
- Golub, L.M.; Lee, H.M.; Ryan, M.E.; Giannobile, W.V.; Payne, J.; Sorsa, T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv. Dent. Res. 1998, 12, 12–26. [Google Scholar] [CrossRef]
- Castro, M.M.; Rizzi, E.; Figueiredo-Lopes, L.; Fernandes, K.; Bendhack, L.M.; Pitol, D.L.; Gerlach, R.F.; Tanus-Santos, J.E. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis 2008, 198, 320–331. [Google Scholar] [CrossRef]
- Guimaraes, D.A.; Rizzi, E.; Ceron, C.S.; Oliveira, A.M.; Oliveira, D.M.; Castro, M.M.; Tirapelli, C.R.; Gerlach, R.F.; Tanus-Santos, J.E. Doxycycline dose-dependently inhibits MMP-2-mediated vascular changes in 2K1C hypertension. Basic Clin. Pharmacol. Toxicol. 2011, 108, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, E.; Castro, M.M.; Prado, C.M.; Silva, C.A.; Fazan, R., Jr.; Rossi, M.A.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix metalloproteinase inhibition improves cardiac dysfunction and remodeling in 2-kidney, 1-clip hypertension. J. Card. Fail. 2010, 16, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Antonio, R.C.; Ceron, C.S.; Rizzi, E.; Coelho, E.B.; Tanus-Santos, J.E.; Gerlach, R.F. Antioxidant effect of doxycycline decreases MMP activity and blood pressure in SHR. Mol. Cell. Biochem. 2014, 386, 99–105. [Google Scholar] [CrossRef]
- Castro, M.M.; Rizzi, E.; Ceron, C.S.; Guimaraes, D.A.; Rodrigues, G.J.; Bendhack, L.M.; Gerlach, R.F.; Tanus-Santos, J.E. Doxycycline ameliorates 2K-1C hypertension-induced vascular dysfunction in rats by attenuating oxidative stress and improving nitric oxide bioavailability. Nitric Oxide Biol. Chem. Off. J. Nitric Oxide Soc. 2012, 26, 162–168. [Google Scholar] [CrossRef]
- Ryan, M.E.; Ashley, R.A. How do tetracyclines work? Adv. Dent. Res. 1998, 12, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.M.; Khalil, R.A. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. Exp. Suppl. 2012, 103, 209–279. [Google Scholar] [CrossRef] [Green Version]
- Arnaboldi, F.; Busnelli, M.; Cornaghi, L.; Manzini, S.; Parolini, C.; Dellera, F.; Ganzetti, G.S.; Sirtori, C.R.; Donetti, E.; Chiesa, G. High-density lipoprotein deficiency in genetically modified mice deeply affects skin morphology: A structural and ultrastructural study. Exp. Cell Res. 2015, 338, 105–112. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, W.; Yancey, P.G.; Koury, M.J.; Zhang, Y.; Fazio, S.; Linton, M.F. Macrophage apolipoprotein E reduces atherosclerosis and prevents premature death in apolipoprotein E and scavenger receptor-class BI double-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Cote, I.; Yasari, S.; Pighon, A.; Barsalani, R.; Rabasa-Lhoret, R.; Prud’homme, D.; Lavoie, J.M. Liver fat accumulation may be dissociated from adiposity gain in ovariectomized rats. Climacteric J. Int. Menopause Soc. 2012, 15, 594–601. [Google Scholar] [CrossRef]
- Meng, Q.; Li, Y.; Ji, T.; Chao, Y.; Li, J.; Fu, Y.; Wang, S.; Chen, Q.; Chen, W.; Huang, F.; et al. Estrogen prevent atherosclerosis by attenuating endothelial cell pyroptosis via activation of estrogen receptor alpha-mediated autophagy. J. Adv. Res. 2021, 28, 149–164. [Google Scholar] [CrossRef]
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Investig. 1994, 94, 2493–2503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galis, Z.S.; Sukhova, G.K.; Libby, P. Microscopic localization of active proteases by in situ zymography: Detection of matrix metalloproteinase activity in vascular tissue. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995, 9, 974–980. [Google Scholar] [CrossRef]
- Vandooren, J.; Geurts, N.; Martens, E.; Van den Steen, P.E.; Opdenakker, G. Zymography methods for visualizing hydrolytic enzymes. Nat. Methods 2013, 10, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yang, G.; Hu, L.; Piao, L.; Inoue, A.; Jiang, H.; Sasaki, T.; Zhao, G.; Yisireyili, M.; Yu, C.; et al. Increased dipeptidyl peptidase-4 accelerates diet-related vascular aging and atherosclerosis in ApoE-deficient mice under chronic stress. Int. J. Cardiol. 2017, 243, 413–420. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Zielke, H.R.; Cheng, L.; Xiao, R.; Crow, M.T.; Stetler-Stevenson, W.G.; Froehlich, J.; Lakatta, E.G. Increased expression of 72-kd type IV collagenase (MMP-2) in human aortic atherosclerotic lesions. Am. J. Pathol. 1996, 148, 121–128. [Google Scholar]
- Wagsater, D.; Zhu, C.; Bjorkegren, J.; Skogsberg, J.; Eriksson, P. MMP-2 and MMP-9 are prominent matrix metalloproteinases during atherosclerosis development in the Ldlr(-/-)Apob(100/100) mouse. Int. J. Mol. Med. 2011, 28, 247–253. [Google Scholar] [CrossRef]
- Heussen, C.; Dowdle, E.B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem. 1980, 102, 196–202. [Google Scholar] [CrossRef]
- Azevedo, A.; Prado, A.F.; Issa, J.P.; Gerlach, R.F. Matrix metalloproteinase 2 fused to GFP, expressed in E. coli, successfully tracked MMP-2 distribution in vivo. Int. J. Biol. Macromol. 2016, 89, 737–745. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, S.J.; Lee, M.Y.; Park, M.W.; Kim, S.S.; Shin, N.; Lovett, D.H.; Bae, S.S.; Ahn, J.; Park, J.S.; et al. Enhanced cardiac expression of two isoforms of matrix metalloproteinase-2 in experimental diabetes mellitus. PLoS ONE 2019, 14, e0221798. [Google Scholar] [CrossRef]
- Marcaccini, A.M.; Novaes, A.B., Jr.; Meschiari, C.A.; Souza, S.L.; Palioto, D.B.; Sorgi, C.A.; Faccioli, L.H.; Tanus-Santos, J.E.; Gerlach, R.F. Circulating matrix metalloproteinase-8 (MMP-8) and MMP-9 are increased in chronic periodontal disease and decrease after non-surgical periodontal therapy. Clin. Chim. Acta Int. J. Clin. Chem. 2009, 409, 117–122. [Google Scholar] [CrossRef]
- Pagliara, V.; De Rosa, M.; Di Donato, P.; Nasso, R.; D’Errico, A.; Cammarota, F.; Poli, A.; Masullo, M.; Arcone, R. Inhibition of Interleukin-6-Induced Matrix Metalloproteinase-2 Expression and Invasive Ability of Lemon Peel Polyphenol Extract in Human Primary Colon Cancer Cells. Molecules 2021, 26, 7076. [Google Scholar] [CrossRef] [PubMed]
- Raisanen, I.T.; Lahteenmaki, H.; Gupta, S.; Grigoriadis, A.; Sahni, V.; Suojanen, J.; Seppanen, H.; Tervahartiala, T.; Sakellari, D.; Sorsa, T. An aMMP-8 Point-of-Care and Questionnaire Based Real-Time Diagnostic Toolkit for Medical Practitioners. Diagnostics 2021, 11, 711. [Google Scholar] [CrossRef]
- Wu, P.S.; Wang, C.Y.; Chen, P.S.; Hung, J.H.; Yen, J.H.; Wu, M.J. 8-Hydroxydaidzein Downregulates JAK/STAT, MMP, Oxidative Phosphorylation, and PI3K/AKT Pathways in K562 Cells. Biomedicines 2021, 9, 1907. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Scarano, F.; Musolino, V.; Carresi, C.; Scicchitano, M.; Ruga, S.; Zito, M.C.; Nucera, S.; Bosco, F.; Maiuolo, J.; et al. Role of TSPO/VDAC1 Upregulation and Matrix Metalloproteinase-2 Localization in the Dysfunctional Myocardium of Hyperglycaemic Rats. Int. J. Mol. Sci. 2020, 21, 7432. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Y.; Lin, R.; Yuan, B.X.; Yang, G.D.; Liu, Y.; Zhang, H. Tanshinone IIA downregulates the CD40 expression and decreases MMP-2 activity on atherosclerosis induced by high fatty diet in rabbit. J. Ethnopharmacol. 2008, 115, 217–222. [Google Scholar] [CrossRef]
- Wu, T.C.; Chen, Y.H.; Leu, H.B.; Chen, Y.L.; Lin, F.Y.; Lin, S.J.; Chen, J.W. Carvedilol, a pharmacological antioxidant, inhibits neointimal matrix metalloproteinase-2 and -9 in experimental atherosclerosis. Free Radic. Biol. Med. 2007, 43, 1508–1522. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Jawien, J.; Toton-Zuranska, J.; Kus, K.; Pawlowska, M.; Olszanecki, R.; Korbut, R. The effect of AVE 0991, nebivolol and doxycycline on inflammatory mediators in an apoE-knockout mouse model of atherosclerosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, BR389–BR393. [Google Scholar] [CrossRef] [Green Version]
- Madan, M.; Bishayi, B.; Hoge, M.; Messas, E.; Amar, S. Doxycycline affects diet- and bacteria-associated atherosclerosis in an ApoE heterozygote murine model: Cytokine profiling implications. Atherosclerosis 2007, 190, 62–72. [Google Scholar] [CrossRef]
- Pawlowska, M.; Gajda, M.; Pyka-Fosciak, G.; Toton-Zuranska, J.; Niepsuj, A.; Kus, K.; Bujak-Gizycka, B.; Suski, M.; Olszanecki, R.; Jawien, J.; et al. The effect of doxycycline on atherogenesis in apoE-knockout mice. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2011, 62, 247–250. [Google Scholar]
- Jung, J.J.; Razavian, M.; Kim, H.Y.; Ye, Y.; Golestani, R.; Toczek, J.; Zhang, J.; Sadeghi, M.M. Matrix metalloproteinase inhibitor, doxycycline and progression of calcific aortic valve disease in hyperlipidemic mice. Sci. Rep. 2016, 6, 32659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, M.W.; Cassis, L.A.; Daugherty, A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riba, A.; Deres, L.; Eros, K.; Szabo, A.; Magyar, K.; Sumegi, B.; Toth, K.; Halmosi, R.; Szabados, E. Doxycycline protects against ROS-induced mitochondrial fragmentation and ISO-induced heart failure. PLoS ONE 2017, 12, e0175195. [Google Scholar] [CrossRef] [PubMed]
- Lovett, D.H.; Mahimkar, R.; Raffai, R.L.; Cape, L.; Maklashina, E.; Cecchini, G.; Karliner, J.S. A novel intracellular isoform of matrix metalloproteinase-2 induced by oxidative stress activates innate immunity. PLoS ONE 2012, 7, e34177. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Akaike, T.; Sawa, T.; Miyamoto, Y.; van der Vliet, A.; Maeda, H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. 2001, 276, 29596–29602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viappiani, S.; Nicolescu, A.C.; Holt, A.; Sawicki, G.; Crawford, B.D.; Leon, H.; van Mulligen, T.; Schulz, R. Activation and modulation of 72kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem. Pharmacol. 2009, 77, 826–834. [Google Scholar] [CrossRef]
- Castro, M.M.; Rizzi, E.; Rodrigues, G.J.; Ceron, C.S.; Bendhack, L.M.; Gerlach, R.F.; Tanus-Santos, J.E. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic. Biol. Med. 2009, 46, 1298–1307. [Google Scholar] [CrossRef]
- Rizzi, E.; Castro, M.M.; Ceron, C.S.; Neto-Neves, E.M.; Prado, C.M.; Rossi, M.A.; Tanus-Santos, J.E.; Gerlach, R.F. Tempol inhibits TGF-beta and MMPs upregulation and prevents cardiac hypertensive changes. Int. J. Cardiol. 2013, 165, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Prado, A.F.; Pernomian, L.; Azevedo, A.; Costa, R.A.P.; Rizzi, E.; Ramos, J.; Paes Leme, A.F.; Bendhack, L.M.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix metalloproteinase-2-induced epidermal growth factor receptor transactivation impairs redox balance in vascular smooth muscle cells and facilitates vascular contraction. Redox Biol. 2018, 18, 181–190. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Meng, X.P.; Ramasamy, S.; Harrison, D.G.; Galis, Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Investig. 1996, 98, 2572–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabek, J.; Glogowska-Ligus, J.; Szadorska, B. Transcription activity of MMP-2 and MMP-9 metalloproteinase genes and their tissue inhibitor (TIMP-2) in acute coronary syndrome patients. J. Postgrad. Med. 2013, 59, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, O.S.; Khan, S.Q.; Narayan, H.K.; Ng, K.H.; Mohammed, N.; Quinn, P.A.; Squire, I.B.; Davies, J.E.; Ng, L.L. Matrix metalloproteinase-2 predicts mortality in patients with acute coronary syndrome. Clin. Sci. 2009, 118, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammann, P.; Rizzoli, R.; Bonjour, J.P.; Bourrin, S.; Meyer, J.M.; Vassalli, P.; Garcia, I. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J. Clin. Investig. 1997, 99, 1699–1703. [Google Scholar] [CrossRef] [PubMed]






| ApoE-⁄-/OVX Vehicle | ApoE-⁄-/OVX Doxycycline | |
|---|---|---|
| Cholesterol total (mg/dL) | 799 ± 48 | 949 ± 47 |
| Cholesterol LDL (mg/dL) | 493 ± 54 | 411 ± 48 |
| Cholesterol HDL (mg/dL) | 5.9 ± 0.3 | 6.0 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, K.E.; Azevedo, A.; Gonçalves, P.R.; Pontes, M.H.B.; Alves, G.M.; Oliveira, R.R.; Amarante, C.B.; Issa, J.P.M.; Gerlach, R.F.; Prado, A.F. Doxycycline Decreases Atherosclerotic Lesions in the Aorta of ApoE-⁄- and Ovariectomized Mice with Correlation to Reduced MMP-2 Activity. Int. J. Mol. Sci. 2022, 23, 2532. https://doi.org/10.3390/ijms23052532
Rodrigues KE, Azevedo A, Gonçalves PR, Pontes MHB, Alves GM, Oliveira RR, Amarante CB, Issa JPM, Gerlach RF, Prado AF. Doxycycline Decreases Atherosclerotic Lesions in the Aorta of ApoE-⁄- and Ovariectomized Mice with Correlation to Reduced MMP-2 Activity. International Journal of Molecular Sciences. 2022; 23(5):2532. https://doi.org/10.3390/ijms23052532
Chicago/Turabian StyleRodrigues, Keuri E., Aline Azevedo, Pricila R. Gonçalves, Maria H. B. Pontes, Gustavo M. Alves, Ruan R. Oliveira, Cristine B. Amarante, João P. M. Issa, Raquel F. Gerlach, and Alejandro F. Prado. 2022. "Doxycycline Decreases Atherosclerotic Lesions in the Aorta of ApoE-⁄- and Ovariectomized Mice with Correlation to Reduced MMP-2 Activity" International Journal of Molecular Sciences 23, no. 5: 2532. https://doi.org/10.3390/ijms23052532
APA StyleRodrigues, K. E., Azevedo, A., Gonçalves, P. R., Pontes, M. H. B., Alves, G. M., Oliveira, R. R., Amarante, C. B., Issa, J. P. M., Gerlach, R. F., & Prado, A. F. (2022). Doxycycline Decreases Atherosclerotic Lesions in the Aorta of ApoE-⁄- and Ovariectomized Mice with Correlation to Reduced MMP-2 Activity. International Journal of Molecular Sciences, 23(5), 2532. https://doi.org/10.3390/ijms23052532