Unsaturated Copolyesters from Macrolactone/Norbornene: Toward Reaction Kinetics of Metathesis Copolymerization Using Ruthenium Carbene Catalysts
Abstract
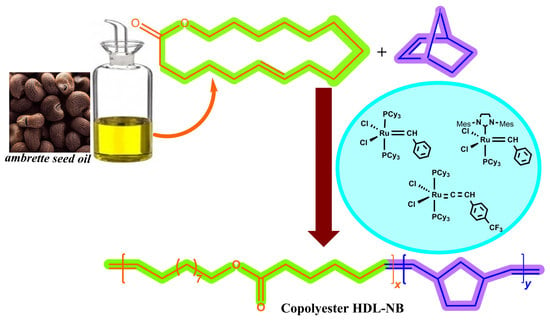
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Characterization Techniques
3.2. General Monomer Polymerization
3.2.1. Synthesis of Polynorbornene (PNB)
3.2.2. Synthesis of Poly(ω-6-Hexadecenlactone) (PHDL)
3.2.3. Synthesis of HDL-NB Copolymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pepels, M.P.F.; Govaert, L.E.; Duchateau, R. Influence of the Main-Chain Configuration on the Mechanical Properties of Linear Aliphatic Polyesters. Macromolecules 2015, 48, 5845–5854. [Google Scholar] [CrossRef]
- Stempfle, F.; Ortmann, P.; Mecking, S. Which polyesters can mimic polyethylene? Macromol. Rapid Commun. 2013, 34, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Hillmyer, M.A. High molar mass poly(ricinoleic acid): Via entropy-driven ring-opening metathesis polymerization. Polym. Chem. 2021, 12, 2253–2257. [Google Scholar] [CrossRef]
- Pepels, M.P.F.; Bouyahyi, M.; Heise, A.; Duchateau, R. Kinetic investigation on the catalytic ring-opening (co)polymerization of (macro)lactones using aluminum salen catalystsIt. Macromolecules 2012, 46, 4324–4334. [Google Scholar] [CrossRef]
- Fuoco, T.; Meduri, A.; Lamberti, M.; Venditto, V.; Pellecchia, C.; Pappalardo, D. Ring-opening polymerization of ω-6-hexadecenlactone by a salicylaldiminato aluminum complex: A route to semicrystalline and functional poly(ester)s. Polym. Chem. 2015, 6, 1727–1740. [Google Scholar] [CrossRef]
- Gong, S.; Du, P.; Ma, H. Binuclear aluminum complexes supported by linked bis(β-diketiminate) ligands for ring-opening polymerization of cyclic esters. Chin. J. Polym. Sci. 2018, 36, 190–201. [Google Scholar] [CrossRef]
- Bouyahyi, M.; Duchateau, R. Metal-Based Catalysts for Controlled Ring-Opening Polymerization of Macrolactones: High Molecular Weight and Well-Defined Copolymer Architectures. Macromolecules 2014, 47, 517–524. [Google Scholar] [CrossRef] [Green Version]
- D’Auria, I.; Santulli, F.; Ciccone, F.; Giannattasio, A.; Mazzeo, M.; Pappalardo, D. Synthesis of Semi-Aromatic Di-Block Polyesters by Terpolymerization of Macrolactones, Epoxides, and Anhydrides. ChemCatChem 2021, 13, 3303–3311. [Google Scholar] [CrossRef]
- Van der Meulen, I.; Li, Y.; Deumens, R.; Joosten, E.A.J.; Koning, C.E.; Heise, A. Copolymers from Unsaturated Macrolactones: Toward the Design of Cross-Linked Biodegradable Polyesters. Biomacromolecules 2011, 12, 837–843. [Google Scholar] [CrossRef]
- Witt, T.; Häußler, M.; Mecking, S. No Strain, No Gain? Enzymatic Ring-Opening Polymerization of Strainless Aliphatic Macrolactones. Macromol. Rapid Commun. 2017, 38, 1600638. [Google Scholar] [CrossRef]
- Tinajero-Díaz, E.; de Ilarduya, A.M.; Muñoz-Guerra, S. Copolymacrolactones Grafted with l-Glutamic Acid: Synthesis, Structure, and Nanocarrier Properties. Polymer 2020, 12, 995. [Google Scholar] [CrossRef]
- Tinajero-Díaz, E.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. Synthesis and properties of diblock copolymers of ω-pentadecalactone and α-amino acids. Eur. Polym. J. 2019, 116, 169–179. [Google Scholar] [CrossRef]
- Martinez, A.; Tlenkopatchev, M.A.; Gutierrez, S. The Unsaturated Polyester Via Ring-Opening Metathesis Polymerization (ROMP) of ω-6-Hexadecenlactone. Curr. Org. Synth. 2018, 15, 566–571. [Google Scholar] [CrossRef]
- Wei, T.; Rempel, G.L.; Pan, Q.-M.; Jiang, B.; Zou, T.-T. A Novel Approval for Degradation of Polybutadiene and Synthesis of Diene-Based Telechelic Oligomers via Olefin Cross Metathesis. Macromol. React. Eng. 2015, 9, 480–489. [Google Scholar] [CrossRef]
- Fürstner, A.; Langemann, K. Conformationally unbiased macrocyclization reactions by ring closing metathesis. J. Org. Chem. 1996, 61, 3942–3943. [Google Scholar] [CrossRef] [Green Version]
- Manzini, B.; Hodge, P.; Ben-Haida, A. Entropically-driven ring-opening polymerization of macrocyclic esters with up to 84-membered rings catalysed by polymer-supported Candida antarctica lipase B. Polym. Chem. 2010, 1, 339–346. [Google Scholar] [CrossRef]
- Hodge, P.; Colquhoun, H.M. Recent work on entropically-driven ring-opening polymerizations: Some potential applications. Polym. Adv. Technol. 2005, 16, 84–94. [Google Scholar] [CrossRef]
- Pepels, M.P.F.; Hansen, M.R.; Goossens, H.; Duchateau, R. From polyethylene to polyester: Influence of ester groups on the physical properties. Macromolecules 2013, 46, 7668–7677. [Google Scholar] [CrossRef]
- Grubbs, R.B.; Grubbs, R.H. 50th Anniversary Perspective: Living Polymerization—Emphasizing the Molecule in Macromolecules. Macromolecules 2017, 50, 6979–6997. [Google Scholar] [CrossRef]
- Yang, J.; Ren, L.; Li, Y. Ring-opening metathesis polymerization of cis-5-norbornene-endo-2,3-dicarboxylic anhydride derivatives using the grubbs third generation catalyst. Chin. J. Polym. Sci. 2017, 35, 36–45. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Grubbs, R.H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007, 32, 1–29. [Google Scholar] [CrossRef]
- Lyapkov, A.; Kiselev, S.; Bozhenkova, G.; Kukurina, O.; Yusubov, M.; Verpoort, F. Ring Opening Metathesis Polymerization. Recent Res. Polym. 2018, 2018, 43. [Google Scholar] [CrossRef] [Green Version]
- Yasir, M.; Liu, P.; Markwart, J.C.; Suraeva, O.; Wurm, F.R.; Smart, M.; Lattuada, J.; Kilbinger, A.F.M. One-Step Ring Opening Metathesis Block-Like Copolymers and their Compositional Analysis by a Novel Retardation Technique. Angew. Chemie Int. Ed. 2020, 59, 13597–13601. [Google Scholar] [CrossRef] [PubMed]
- Gringolts, M.L.; Denisova, Y.I.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Olefin metathesis in multiblock copolymer synthesis, Beilstein. J. Org. Chem. 2019, 15, 218–235. [Google Scholar] [CrossRef]
- Fernandes, H.; Filgueiras, J.G.; de Azevedo, E.R.; Lima-Neto, B.S. Real time monitoring by time-domain NMR of ring opening metathesis copolymerization of norbornene-based red palm olein monomer with norbornene. Eur. Polym. J. 2020, 140, 110048. [Google Scholar] [CrossRef]
- Paradiso, V.; Grisi, F. Ruthenium-Catalyzed Alternating Ring-Opening Metathesis Copolymerization of Norborn-2-ene with Cyclic Olefins. Adv. Synth. Catal. 2019, 361, 4133–4139. [Google Scholar] [CrossRef]
- Vasiuta, R.; Stockert, A.; Plenio, H. Alternating ring-opening metathesis polymerization by Grubbs-type catalysts with: N-pentiptycenyl, N-alkyl-NHC ligands. Chem. Commun. 2018, 54, 1706–1709. [Google Scholar] [CrossRef]
- Song, A.; Parker, K.A.; Sampson, N.S. Synthesis of Copolymers by Alternating ROMP (AROMP). J. Am. Chem. Soc. 2009, 131, 3444–3445. [Google Scholar] [CrossRef] [Green Version]
- Yasir, M.; Kilbinger, A.F.M. Cascade Ring-Opening/Ring-Closing Metathesis Polymerization of a Monomer Containing a Norbornene and a Cyclohexene Ring. ACS Macro Lett. 2021, 10, 210–214. [Google Scholar] [CrossRef]
- Gringolts, M.L.; Denisova, Y.I.; Shandryuk, G.A.; Krentsel, L.B.; Litmanovich, A.D.; Finkelshtein, E.S.; Kudryavtsev, Y.V. Synthesis of norbornene-cyclooctene copolymers by the cross-metathesis of polynorbornene with polyoctenamer. RSC Adv. 2015, 5, 316–319. [Google Scholar] [CrossRef]
- Gutekunst, W.R.; Hawker, C.J. A General Approach to Sequence-Controlled Polymers Using Macrocyclic Ring Opening Metathesis Polymerization. J. Am. Chem. Soc. 2015, 137, 8038–8041. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.L.; Guo, L.X.; Lin, B.P.; Zhang, X.Q.; Sun, Y.; Yang, H. An entropy-driven ring-opening metathesis polymerization approach towards main-chain liquid crystalline polymers. Polym. Chem. 2016, 7, 5265–5272. [Google Scholar] [CrossRef]
- Xue, Z.; Mayer, M.F. Entropy-driven ring-opening olefin metathesis polymerizations of macrocycles. Soft Matter. 2009, 5, 4600–4611. [Google Scholar] [CrossRef]
- Pearce, A.K.; Foster, J.C.; O’Reilly, R.K. Recent developments in entropy-driven ring-opening metathesis polymerization: Mechanistic considerations, unique functionality, and sequence control. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1621–1634. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Swager, T.M. Main-chain calix[4]arene elastomers by ring-opening metathesis polymerization. Macromolecules 2007, 40, 7437–7440. [Google Scholar] [CrossRef]
- Martínez, A.; Clark-Tapia, R.; Gutierrez, S.; Tlenkopatchev, M. Synthesis and Characterization of New Ruthenium Vinylidene Complexes. Lett. Org. Chem. 2014, 11, 748–754. [Google Scholar] [CrossRef]
- Justin, R.; Griffiths, J.R.; Diver, S.T. Factors Affecting Initiation Rates. In Handbook of Metathesis, 2nd ed.; Grubbs, R.H., Wenzel, A.G., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Berlin, Germany, 2015; Volume 2, pp. 273–279. [Google Scholar] [CrossRef]
- Mayo, F.R.; Lewis, F.M.; Copolymerization, I. A Basis for Comparing the Behavior of Monomers in Copolymerization; The Copolymerization of Styrene and Methyl Methacrylate. J. Am. Chem. Soc. 1944, 66, 1594–1601. [Google Scholar] [CrossRef]
- Fineman, M.; Ross, S.D. Linear Method for Determining Monomer Reactivity Ratios in Copolymerization. J. Polym. Sci. 1950, 5, 259–262. [Google Scholar] [CrossRef]
- Erbil, C.; Özdemir, S.; Uyanik, N. Determination of the monomer reactivity ratios for copolymerization of itaconic acid and acrylamide by conductometric titration method. Polymer 2000, 41, 1391–1394. [Google Scholar] [CrossRef]
- Schleyer, P.V.R.; Wiliiams, J.E.; Blanchard, K.R. Evaluation of strain in hydrocarbons. The strain in adamantane and its origin. J. Am. Chem. Soc. 1970, 92, 2377–2386. [Google Scholar] [CrossRef]
- Hlil, A.R.; Balogh, J.; Moncho, S.; Su, H.L.; Tuba, R.; Brothers, E.N.; Al-Hashimi, M.; Bazzi, H.S. Ring opening metathesis polymerization (ROMP) of five- to eight-membered cyclic olefins: Computational, thermodynamic, and experimental approach. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3137–3145. [Google Scholar] [CrossRef] [Green Version]
- Hodge, P. Entropically driven ring-opening polymerization of strainless organic macrocycles. Chem. Rev. 2014, 114, 2278–2312. [Google Scholar] [CrossRef] [PubMed]





| Entry a | Molar Ratio b | [Ru] | [C=C] c [Ru] | Time (h) | Temp (°C) | Yield (%) d | Mne (g mol−1) | MWD e | HDL/NB Expected wt. % | HDL/NB Measured f wt. % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HDL NB | |||||||||||
| 1 | 1 | 0 | Ru1 | 500 | 3 | 50 | 96 | 109,600 | 1.15 | -- | -- |
| 2 | 0 | 1 | Ru1 | 1000 | 40 min | 25 | 99 | 134,000 | 1.10 | -- | -- |
| 3 | 1 | 1 | Ru1 | 500 | 4 min | 50 | 22 | 78,876 | 2.80 | -- | 39/61 |
| 4 | 1 | 1 | Ru1 | 500 | 30 min | 50 | 36 | 81,500 | 2.90 | -- | 42/58 |
| 5 | 1 | 1 | Ru1 | 500 | 3 | 50 | 49 | 88,677 | 2.78 | -- | 57/43 |
| 6 | 1 | 1 | Ru1 | 500 | 8 | 50 | 72 | 91,981 | 2.84 | -- | 61/39 |
| 7 | 1 | 1 | Ru1 | 500 | 20 | 50 | 97 | 104,424 | 2.10 | 74/26 | 72/28 |
| 8 | 1 | 1 | Ru2 | 250 | 20 | 50 | 66 | 94,210 | 2.00 | 74/26 | 70/30 |
| 9 | 1 | 1 | Ru3 | 250 | 20 | 80 | 57 | 92,005 | 2.09 | 74/26 | 69/31 |
| 10 | 1 | 5 | Ru1 | 500 | 20 | 50 | 96 | 110,500 | 2.20 | 35/65 | 32/68 |
| 11 | 1 | 10 | Ru1 | 500 | 20 | 50 | 96 | 118,080 | 2.00 | 20/80 | 17/83 |
| 12 | 2 | 1 | Ru1 | 500 | 20 | 50 | 98 | 114,600 | 2.10 | 84/16 | 82/18 |
| 13 | 2 | 1 | Ru2 | 250 | 20 | 50 | 53 | 90,600 | 2.10 | 84/16 | 79/21 |
| 14 | 2 | 1 | Ru3 | 250 | 20 | 80 | 42 | 88,500 | 2.38 | 84/16 | 77/23 |
| 15 | 3 | 1 | Ru1 | 500 | 20 | 50 | 97 | 115,000 | 2.10 | 89/11 | 87/13 |
| 16 | 10 | 1 | Ru1 | 500 | 20 | 50 | 98 | 113,790 | 2.00 | 91/9 | 89/11 |
| Entry | Mass of HDL in the Feed (g) | [HDL] a [NB] | Mol % of HDL in the Feed b | Incorporation of HDL in Copolymer (%) c | Time (h) | Yield d (%) |
|---|---|---|---|---|---|---|
| Second-Generation Grubbs (Ru1) | ||||||
| 1 | 0.48 | 1:1 | 50 | 39.00 | 4 min | 22.10 |
| 2 | 0.48 | 1.5:1 | 60 | 48.00 | 4 min | 19.60 |
| 3 | 0.48 | 2:1 | 67 | 62.00 | 6 min | 16.50 |
| 4 | 0.48 | 3:1 | 75 | 74.00 | 6 min | 8.40 |
| 5 | 0.48 | 10:1 | 91 | 86.00 | 10 min | 5.30 |
| First-Generation Grubbs (Ru2) | ||||||
| 6 | 0.48 | 1:1 | 50 | 35.40 | 2 | 19.50 |
| 7 | 0.48 | 1.5:1 | 60 | 42.90 | 2 | 15.40 |
| 8 | 0.48 | 2:1 | 67 | 48.00 | 4 | 13.40 |
| 9 | 0.48 | 3:1 | 75 | 61.30 | 4 | 10.20 |
| 10 | 0.48 | 10:1 | 91 | 78.90 | 7 | 7.60 |
| First-Generation Vinylidene (Ru3) | ||||||
| 11 | 0.48 | 1:1 | 50 | 27.50 | 2 | 15.70 |
| 12 | 0.48 | 1.5:1 | 60 | 43.40 | 2 | 13.30 |
| 13 | 0.48 | 2:1 | 67 | 47.10 | 4 | 12.30 |
| 14 | 0.48 | 3:1 | 75 | 59.40 | 4 | 10.10 |
| 15 | 0.48 | 10:1 | 91 | 77.60 | 7 | 7.20 |
| Mayo–Lewis Method | Finemann–Ross Method | |||
|---|---|---|---|---|
| Catalyst | rHDA | rNB | rHDA | rNB |
 | 0.10 | 5.60 | 0.12 | 5.81 |
 | 0.24 | 3.78 | 0.28 | 4.02 |
 | 0.06 | 4.47 | 0.07 | 4.30 |
| Entry | Molar Ratio a | Thermal Properties | Crystallinity X-ray e | Mechanical Properties f | |||||
|---|---|---|---|---|---|---|---|---|---|
| HDL | NB | Tm b (°C) | Td c (°C) | ΔHm d (J/g) | (%) | E (MPa) | σ (MPa) | ε (%) | |
| 1 | 0 | 1 | -- | 418 | -- | -- | 1280 | 35.60 | 4.00 |
| 2 | 1 | 0 | 47.60 | 384 | 73.00 | 31.00 | 119 | 4.84 | 12.50 |
| 3 | 10 | 1 | 44.34 | 390 | 53.50 | 26.50 | 156 | 7.55 | 10.47 |
| 4 | 2 | 1 | 42.10 | 397 | 47.10 | 23.00 | 229 | 8.13 | 8.637 |
| 5 | 1 | 1 | 40.50 | 400 | 43.30 | 19.80 | 464 | 13.73 | 6.40 |
| 6 | 1 | 5 | 38.10 | 409 | 23.20 | 15.90 | 695 | 19.01 | 5.87 |
| 7 | 1 | 10 | 37.20 | 411 | 12.00 | -- | 775 | 26.33 | 4.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, A.; Zárate-Saldaña, D.; Vargas, J.; Santiago, A.A. Unsaturated Copolyesters from Macrolactone/Norbornene: Toward Reaction Kinetics of Metathesis Copolymerization Using Ruthenium Carbene Catalysts. Int. J. Mol. Sci. 2022, 23, 4521. https://doi.org/10.3390/ijms23094521
Martínez A, Zárate-Saldaña D, Vargas J, Santiago AA. Unsaturated Copolyesters from Macrolactone/Norbornene: Toward Reaction Kinetics of Metathesis Copolymerization Using Ruthenium Carbene Catalysts. International Journal of Molecular Sciences. 2022; 23(9):4521. https://doi.org/10.3390/ijms23094521
Chicago/Turabian StyleMartínez, Araceli, Daniel Zárate-Saldaña, Joel Vargas, and Arlette A. Santiago. 2022. "Unsaturated Copolyesters from Macrolactone/Norbornene: Toward Reaction Kinetics of Metathesis Copolymerization Using Ruthenium Carbene Catalysts" International Journal of Molecular Sciences 23, no. 9: 4521. https://doi.org/10.3390/ijms23094521
APA StyleMartínez, A., Zárate-Saldaña, D., Vargas, J., & Santiago, A. A. (2022). Unsaturated Copolyesters from Macrolactone/Norbornene: Toward Reaction Kinetics of Metathesis Copolymerization Using Ruthenium Carbene Catalysts. International Journal of Molecular Sciences, 23(9), 4521. https://doi.org/10.3390/ijms23094521