Polyphenols as Drivers of a Homeostatic Gut Microecology and Immuno-Metabolic Traits of Akkermansia muciniphila: From Mouse to Man
Abstract
:1. Introduction
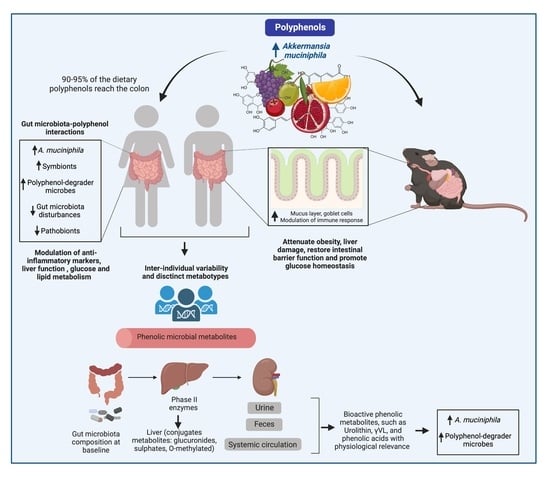
2. Akkermansia muciniphila, the Gut Microenvironment, and Immune Regulation
3. Interactions between Polyphenols and the Gut Microbiota and Their Impact on the Attenuation of Chronic Diseases
4. Modulation of A. muciniphila by Polyphenol-Rich Foods In Vitro and in Animal Models
4.1. Anthocyanin-Rich Foods
4.2. Flavan-3-Nol-Rich Foods
4.3. Flavonol-Rich Foods
4.4. Flavanone and Flavonone-Rich Foods
4.5. Phenolic Acid-Rich Foods
4.6. Stilbene-Rich Foods
4.7. Lignan-Rich Foods
5. Modulation of A. muciniphila by Polyphenol-Rich Foods in Human Intervention Trials
6. Indirect Mechanism Favoring A. muciniphila: Polyphenol Signaling of the Host Gut Epithelium and Influencing the Gut Microbial Environment
6.1. Goblet Cells and Mucin Differentiation
6.2. Aryl Hydrocarbon Receptor (AhR) and Gut Inflammation
6.3. Inhibitory Action on Gut Microbial Competitors
7. Polyphenol Effects on A. muciniphila Fitness: Hints of Metabolism and Adaptation to Polyphenol-Rich Foods
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajilic-Stojanovic, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Preidis, G.A.; Shulman, R.; Kashyap, P.C. The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Egea-Zorrilla, A.; Plaza-Diaz, J.; Aragon-Vela, J.; Munoz-Quezada, S.; Tercedor-Sanchez, L.; Abadia-Molina, F. The gut microbiota and its implication in the development of atherosclerosis and related cardiovascular diseases. Nutrients 2020, 12, 605. [Google Scholar] [CrossRef] [Green Version]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut microbiome and liver diseases. Gut 2016, 65, 2035–2044. [Google Scholar] [CrossRef]
- Powell, N.; Walker, M.M.; Talley, N.J. The mucosal immune system: Master regulator of bidirectional gut-brain communications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 143–159. [Google Scholar] [CrossRef]
- Quigley, E.M.M. The gut-brain axis and the microbiome: Clues to pathophysiology and opportunities for novel management strategies in irritable bowel syndrome (IBS). J. Clin. Med. 2018, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Brahe, L.K.; Le Chatelier, E.; Prifti, E.; Pons, N.; Kennedy, S.; Hansen, T.; Pedersen, O.; Astrup, A.; Ehrlich, S.D.; Larsen, L.H. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr. Diabetes 2015, 5, e159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yassour, M.; Lim, M.Y.; Yun, H.S.; Tickle, T.L.; Sung, J.; Song, Y.M.; Lee, K.; Franzosa, E.A.; Morgan, X.C.; Gevers, D.; et al. Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes. Genome Med. 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2016, 23, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Everard, A.; Plovier, H.; Rastelli, M.; Van Hul, M.; de Wouters d’Oplinter, A.; Geurts, L.; Druart, C.; Robine, S.; Delzenne, N.M.; Muccioli, G.G.; et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat. Commun. 2019, 10, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Liu, W.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Liu, R.; Hong, J. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J. Mol. Endocrinol. 2017, 58, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, A.C.; Fernandes, G.R.; da Silva, I.T.; Almeida-Pititto, B.; Gomes, E.P.; Pereira, A.D.; Ferreira, S.R. Enterotype may drive the dietary-associated cardiometabolic risk factors. Front. Cell Infect. Microbiol. 2017, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Costea, P.I.; Hildebrand, F.; Arumugam, M.; Backhed, F.; Blaser, M.J.; Bushman, F.D.; de Vos, W.M.; Ehrlich, S.D.; Fraser, C.M.; Hattori, M.; et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 2018, 3, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Mastrodonato, M.; Calamita, G.; Mentino, D.; Scillitani, G. High-fat diet alters the glycosylation patterns of duodenal mucins in a murine model. J. Histochem. Cytochem. 2020, 68, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Mastrodonato, M.; Mentino, D.; Portincasa, P.; Calamita, G.; Liquori, G.E.; Ferri, D. High-fat diet alters the oligosaccharide chains of colon mucins in mice. Histochem. Cell Biol. 2014, 142, 449–459. [Google Scholar] [CrossRef]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High fat diets Iiduce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergstrom, K.; Shan, X.; Casero, D.; Batushansky, A.; Lagishetty, V.; Jacobs, J.P.; Hoover, C.; Kondo, Y.; Shao, B.; Gao, L.; et al. Proximal colon-derived O-glycosylated mucus encapsulates and modulates the microbiota. Science 2020, 370, 467–472. [Google Scholar] [CrossRef]
- Liu, H.Y.; Walden, T.B.; Ahl, D.; Nyman, M.; Bertilsson, S.; Phillipson, M.; Holm, L. High-fat diet enriched with bilberry modifies colonic mucus dynamics and restores marked alterations of gut microbiome in rats. Mol. Nutr. Food Res. 2019, 63, e1900117. [Google Scholar] [CrossRef]
- Forgie, A.J.; Ju, T.; Tollenaar, S.L.; Willing, B.P. Phytochemical-induced mucin accumulation in the gastrointestinal lumen is independent of the microbiota. bioRxiv 2022. bioRxiv:2022.03.11.483917. [Google Scholar]
- Makarewicz, M.; Drozdz, I.; Tarko, T.; Duda-Chodak, A. The Interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Truchado, P.; Larrosa, M.; Espín, J.C.; Tomás-Barberán, F.A.; Allende, A.; García-Conesa, M.T. Urolithins, ellagitannin metabolites produced by colon microbiota, inhibit quorum sensing in Yersinia Enterocolitica: Phenotypic response and associated molecular changes. Food Chem 2012, 132, 1465–1474. [Google Scholar] [CrossRef]
- Saha, P.; Yeoh, B.; Singh, R.; Chandrasekar, B.; Vemula, P.; Haribabu, B.; Vijay-Kumar, M.; Jala, V.R. Gut Microbiota Conversion of Dietary Ellagic Acid into Bioactive Phytoceutical Urolithin A Inhibits Heme Peroxidases. PLoS ONE 2016, 11, e0156811. [Google Scholar] [CrossRef] [Green Version]
- Mele, L.; Carobbio, S.; Brindani, N.; Curti, C.; Rodriguez-Cuenca, S.; Bidault, G.; Mena, P.; Zanotti, I.; Vacca, M.; Vidal-Puig, A.; et al. Phenyl-gamma-valerolactones, flavan-3-ol colonic metabolites, protect brown adipocytes from oxidative stress without affecting their differentiation or function. Mol. Nutr. Food Res. 2017, 61, 1700074. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2018, 36, 714–752. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. In vitro bioaccessibility, human gut microbiota metabolites and hepatoprotective potential of Chebulic ellagitannins: A case of padma hepaten(R) formulation. Nutrients 2015, 7, 8456–8477. [Google Scholar] [CrossRef] [Green Version]
- Karn, A.; Zhao, C.; Yang, F.; Cui, J.; Gao, Z.; Wang, M.; Wang, F.; Xiao, H.; Zheng, J. In-vivo biotransformation of citrus functional components and their effects on health. Crit. Rev. Food Sci. Nutr. 2021, 61, 756–776. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottaviani, J.I.; Borges, G.; Momma, T.Y.; Spencer, J.P.; Keen, C.L.; Crozier, A.; Schroeter, H. The metabolome of [2-(14)C](-)-epicatechin in humans: Implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci Rep. 2016, 6, 29034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, H.; Wu, S.; Sakao, K.; Hara, T.; He, J.; Garcia, S.; Shetty, K.; Hou, D.X. Bilberry anthocyanins ameliorate NAFLD by improving dyslipidemia and gut microbiome dysbiosis. Nutrients 2020, 12, 3252. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Juárez-Fernández, M.; Porras, D.; Petrov, P.; Román-Sagüillo, S.; García-Mediavilla, M.V.; Soluyanova, P.; Martínez-Flórez, S.; González-Gallego, J.; Nistal, E.; Jover, R.; et al. The Synbiotic Combination of Akkermansia muciniphila and Quercetin Ameliorates Early Obesity and NAFLD through Gut Microbiota Reshaping and Bile Acid Metabolism Modulation. Antioxidants 2021, 10, 2001. [Google Scholar] [CrossRef]
- Fidelix, M.; Milenkovic, D.; Sivieri, K.; Cesar, T. Microbiota modulation and effects on metabolic biomarkers by orange juice: A controlled clinical trial. Food Funct. 2020, 11, 1599–1610. [Google Scholar] [CrossRef]
- Anhê, F.F.; Varin, T.V.; Barz, M.; Pilon, G.; Dudonné, S.; Trottier, J.; St-Pierre, P.; Harris, C.S.; Lucas, M.; Lemire, M.; et al. Arctic berry extracts target the gut–liver axis to alleviate metabolic endotoxaemia, insulin resistance and hepatic steatosis in diet-induced obese mice. Diabetologia 2018, 61, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Dunngalvin, G.; Kern, T.; Blanco-Bose, W.; Auwerx, J.; Aebischer, P.; Rinsch, C. Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur. J. Clin. Nutr. 2022, 76, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Summanen, P.H.; Lee, R.-P.; Yang, J.; Finegold, S.M.; Heber, D.; Li, Z. Pomegranate ellagitannins stimulate the growth of Akkermansia muciniphila in vivo. Anaerobe 2017, 43, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-mediated gut microbiota modulation: Toward prebiotics and further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Chen, H.J.; Xie, G.R.; Shih, C.K. Djulis (Chenopodium Formosanum) prevents colon carcinogenesis via regulating antioxidative and apoptotic pathways in rats. Nutrients 2019, 11, 2168. [Google Scholar] [CrossRef] [Green Version]
- Van Dorsten, F.A.; Peters, S.; Gross, G.; Gomez-Roldan, V.; Klinkenberg, M.; de Vos, R.C.; Vaughan, E.E.; van Duynhoven, J.P.; Possemiers, S.; van de Wiele, T.; et al. Gut microbial metabolism of polyphenols from black tea and red wine/grape juice is source-specific and colon-region dependent. J. Agric. Food Chem. 2012, 60, 11331–11342. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Q.; Ma, Y.; Li, L.; Yu, K.; Zhang, Z.; Chen, G.; Li, X.; Xiao, W.; Xu, P.; et al. Aryl hydrocarbon receptor activation modulates intestinal epithelial barrier function by maintaining tight junction integrity. Int. J. Biol. Sci. 2018, 14, 69–77. [Google Scholar] [CrossRef]
- Xue, Z.; Li, D.; Yu, W.; Zhang, Q.; Hou, X.; He, Y.; Kou, X. Mechanisms and therapeutic prospects of polyphenols as modulators of the aryl hydrocarbon receptor. Food Funct. 2017, 8, 1414–1437. [Google Scholar] [CrossRef]
- Tang, J.S.; Cait, A.; Li, Y.; Abolins-Thompson, H.; Gell, K.; Herst, P.M.; O’Sullivan, D.; Gasser, O. Practical approach to explore the effects of polyphenols on aryl hydrocarbon receptor regulated immune function. J. Agric. Food Chem. 2021, 69, 8625–8633. [Google Scholar] [CrossRef]
- Bachmann, R.; Van Hul, M.; Baldin, P.; Léonard, D.; Delzenne, N.M.; Belzer, C.; Ouwerkerk, J.P.; Repsilber, D.; Rangel, I.; Kartheuser, A.; et al. Akkermansia muciniphila reduces peritonitis and improves intestinal tissue wound healing after a colonic transmural defect by a MyD88-dependent mechanism. Cells 2022, 11, 2666. [Google Scholar] [CrossRef]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Henning, S.M.; Lee, R.P.; Lu, Q.Y.; Summanen, P.H.; Thames, G.; Corbett, K.; Downes, J.; Tseng, C.H.; Finegoldbce, S.M.; et al. Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food Funct. 2015, 6, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Villalba, R.; Vissenaekens, H.; Pitart, J.; Romo-Vaquero, M.; Espín, J.; Grootaert, C.; Selma, M.V.; Raes, K.; Smagghe, G.; Possemiers, S.; et al. The gastrointestinal simulation model TWIN-SHIME® Shows differences between human Urolithin-metabotypes in gut microbiota composition, pomegranate polyphenol metabolism, and transport along the intestinal tract. J. Agric. Food Chem. 2017, 65, 5480–5493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Zhang, X.; Jiang, M.; Zhang, H.; Wang, Y.; Zhang, Y.; Seviour, R.; Kong, Y. In vitro co-metabolism of epigallocatechin-3-gallate (EGCG) by the mucin-degrading bacterium Akkermansia muciniphila. PLoS ONE 2021, 16, e0260757. [Google Scholar] [CrossRef]
- Johansson, M.E.; Larsson, J.M.; Hansson, G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4659–4665. [Google Scholar] [CrossRef] [Green Version]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid. Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- van Passel, M.W.J.; Kant, R.; Zoetendal, E.G.; Plugge, C.M.; Derrien, M.; Malfatti, S.A.; Chain, P.S.G.; Woyke, T.; Palva, A.; de Vos, W.M.; et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS ONE 2011, 6, e16876, 1-8. [Google Scholar] [CrossRef] [Green Version]
- Ottman, N.; Davids, M.; Suarez-Diez, M.; Boeren, S.; Schaap, P.J.; Martins Dos Santos, V.A.P.; Smidt, H.; Belzer, C.; de Vos, W.M. Genome-scale model and omics analysis of metabolic capacities of Akkermansia muciniphila reveal a preferential mucin-degrading lifestyle. Appl. Environ. Microbiol. 2017, 83, e01014-17. [Google Scholar] [CrossRef] [Green Version]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Earley, H.; Lennon, G.; Balfe, A.; Coffey, J.C.; Winter, D.C.; O’Connell, P.R. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci. Rep. 2019, 9, 15683. [Google Scholar] [CrossRef]
- Yamada, T.; Hino, S.; Iijima, H.; Genda, T.; Aoki, R.; Nagata, R.; Han, K.H.; Hirota, M.; Kinashi, Y.; Oguchi, H.; et al. Mucin O-glycans facilitate symbiosynthesis to maintain gut immune homeostasis. EBioMedicine 2019, 48, 513–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, J.M.; Karlsson, H.; Crespo, J.G.; Johansson, M.E.; Eklund, L.; Sjovall, H.; Hansson, G.C. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 2011, 17, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Giron, L.B.; Tanes, C.E.; Schleimann, M.H.; Engen, P.A.; Mattei, L.M.; Anzurez, A.; Damra, M.; Zhang, H.; Bittinger, K.; Bushman, F.; et al. Sialylation and fucosylation modulate inflammasome-activating eIF2 Signaling and microbial translocation during HIV infection. Mucosal. Immunol. 2020, 13, 753–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruss, K.M.; Marcobal, A.; Southwick, A.M.; Dahan, D.; Smits, S.A.; Ferreyra, J.A.; Higginbottom, S.K.; Sonnenburg, E.D.; Kashyap, P.C.; Choudhury, B.; et al. Mucin-derived O-glycans supplemented to diet mitigate diverse microbiota perturbations. ISME J. 2021, 15, 577–591. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Goto, Y.; Obata, T.; Kunisawa, J.; Sato, S.; Ivanov, I.I.; Lamichhane, A.; Takeyama, N.; Kamioka, M.; Sakamoto, M.; Matsuki, T.; et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 2014, 345, 1254009. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef]
- Takamura, T.; Harama, D.; Fukumoto, S.; Nakamura, Y.; Shimokawa, N.; Ishimaru, K.; Ikegami, S.; Makino, S.; Kitamura, M.; Nakao, A. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol. Cell Biol. 2011, 89, 817–822. [Google Scholar] [CrossRef]
- Yin, J.; Song, Y.; Hu, Y.; Wang, Y.; Zhang, B.; Wang, J.; Ji, X.; Wang, S. Dose-dependent beneficial effects of tryptophan and its derived metabolites on Akkermansia In Vitro: A preliminary prospective study. Microorganisms 2021, 9, 1511. [Google Scholar] [CrossRef]
- Gu, Z.; Pei, W.; Shen, Y.; Wang, L.; Zhu, J.; Zhang, Y.; Fan, S.; Wu, Q.; Li, L.; Zhang, Z. Akkermansia muciniphila and its outer protein Amuc_1100 regulates tryptophan metabolism in colitis. Food Funct. 2021, 12, 10184–10195. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Dietary polyphenols: A novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci. Technol. 2018, 78, 224–233. [Google Scholar] [CrossRef]
- Gao, X.; Xie, Q.; Kong, P.; Liu, L.; Sun, S.; Xiong, B.; Huang, B.; Yan, L.; Sheng, J.; Xiang, H. Polyphenol- and caffeine-rich postfermented Pu-erh tea improves diet-induced metabolic syndrome by remodeling intestinal homeostasis in mice. Infect. Immun. 2018, 86, e00601-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhao, Y.; Ohland, C.; Jobin, C.; Sang, S. Microbiota facilitates the formation of the aminated metabolite of green tea polyphenol (-)-epigallocatechin-3-gallate which trap deleterious reactive endogenous metabolites. Free Radic. Biol. Med. 2019, 131, 332–344. [Google Scholar] [CrossRef]
- Hara-Terawaki, A.; Takagaki, A.; Kobayashi, H.; Nanjo, F. Inhibitory activity of catechin metabolites produced by intestinal microbiota on proliferation of HeLa cells. Biol. Pharm. Bull. 2017, 40, 1331–1335. [Google Scholar] [CrossRef] [Green Version]
- Takagaki, A.; Nanjo, F. Effects of metabolites produced from (-)-epigallocatechin gallate by rat intestinal bacteria on angiotensin I-converting enzyme activity and blood pressure in spontaneously hypertensive rats. J. Agric. Food Chem 2015, 63, 8262–8266. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Boeres, A.; Weber, T.; Dos Santos, C.N.; Ventura, M.R.; Heiss, C. Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study. Mol. Nutr. Food Res. 2016, 60, 2130–2140. [Google Scholar] [CrossRef] [Green Version]
- Mi, Y.; Qi, G.; Gao, Y.; Li, R.; Wang, Y.; Li, X.; Huang, S.; Liu, X. (-)-Epigallocatechin-3-gallate ameliorates insulin resistance and mitochondrial dysfunction in HepG2 cells: Involvement of Bmal1. Mol. Nutr. Food Res. 2017, 61, 1700440. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of gut microbiota, microbial metabolites and interaction with polyphenol in host immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, microbiome and health benefits in aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef] [PubMed]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.R.; Liu, X.M.; Chen, Z.Y.; Zhang, Y.S.; Zhang, Y.H. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chem. 2016, 213, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Dabek, M.; McCrae, S.I.; Stevens, V.J.; Duncan, S.H.; Louis, P. Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 2008, 66, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Shen, X.; Deng, R.; Zhang, Y.; Zheng, X. Dietary anthocyanin-rich extract of acai protects from diet-induced obesity, liver steatosis, and insulin resistance with modulation of gut microbiota in mice. Nutrition 2021, 86, 111176. [Google Scholar] [CrossRef]
- John, O.D.; Mouatt, P.; Prasadam, I.; Xiao, Y.; Panchal, S.K.; Brown, L. The edible native Australian fruit, Davidson’s plum (Davidsonia pruriens), reduces symptoms in rats with diet-induced metabolic syndrome. J. Funct. Foods 2019, 56, 204–215. [Google Scholar] [CrossRef]
- Pan, P.; Lam, V.; Salzman, N.; Huang, Y.-W.; Yu, J.; Zhang, J.; Wang, L.-S. Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F-344 rats. Nutr. Cancer. 2017, 69, 943–951. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; Hou, D.X.; Wu, S. The effects and mechanisms of cyanidin-3-glucoside and its phenolic metabolites in maintaining intestinal integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Ajiboye, T.O.; Habibu, R.S.; Saidu, K.; Haliru, F.Z.; Ajiboye, H.O.; Aliyu, N.O.; Ibitoye, O.B.; Uwazie, J.N.; Muritala, H.F.; Bello, S.A.; et al. Involvement of oxidative stress in protocatechuic acid-mediated bacterial lethality. Microbiologyopen 2017, 6, e00472. [Google Scholar] [CrossRef]
- Esteban-Torres, M.; Santamaria, L.; Cabrera-Rubio, R.; Plaza-Vinuesa, L.; Crispie, F.; de Las Rivas, B.; Cotter, P.; Munoz, R. A diverse range of human gut bacteria have the potential to metabolize the dietary component gallic acid. Appl. Environ. Microbiol. 2018, 84, e01558-18. [Google Scholar] [CrossRef]
- Chia, L.; Hornung, B.V.H.; Aalvink, S.; Schaap, P.J.; de Vos, W.M.; Knol, J.; Belzer, C. Deciphering the trophic interaction between Akkermansia muciniphila and the butyrogenic gut commensal Anaerostipes caccae using a metatranscriptomic approach. Antonie Van Leeuwenhoek 2018, 111, 859–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Jian, Y.; Liu, Y.; Jiang, S.; Muhammad, D.; Wang, W. Flavanols from nature: A phytochemistry and biological activity review. Molecules 2022, 27, 719. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.C. Nutrition epidemiology of flavan-3-ols: The known unknowns. Mol. Asp. Med. 2018, 61, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sang, S. Biotransformation of tea polyphenols by gut microbiota. J. Funct. Foods 2014, 7, 26–42. [Google Scholar] [CrossRef]
- Kan, J.; Chen, C.; Huo, T.; Xie, W.; Hui, Y.; Liu, J.; Jin, C. Polyphenolic-enriched peach peels extract regulates lipid metabolism and improves the gut microbiota composition in high fat diet-fed mice. J. Funct. Foods 2020, 72, 104082. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [Green Version]
- Farha, A.K.; Yang, Q.-Q.; Kim, G.; Li, H.-B.; Zhu, F.; Liu, H.-Y.; Gan, R.-Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Rodriguez-Daza, M.C.; Daoust, L.; Boutkrabt, L.; Pilon, G.; Varin, T.; Dudonne, S.; Levy, E.; Marette, A.; Roy, D.; Desjardins, Y. Wild blueberry proanthocyanidins shape distinct gut microbiota profile and influence glucose homeostasis and intestinal phenotypes in high-fat high-sucrose fed mice. Sci. Rep. 2020, 10, 2217. [Google Scholar] [CrossRef] [Green Version]
- Neto, C.C.; Mortzfeld, B.M.; Turbitt, J.R.; Bhattarai, S.K.; Yeliseyev, V.; DiBenedetto, N.; Bry, L.; Bucci, V. Proanthocyanidin-enriched cranberry extract induces resilient bacterial community dynamics in a gnotobiotic mouse model. Microb. Cell 2021, 8, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Daza, M.C.; Roquim, M.; Dudonne, S.; Pilon, G.; Levy, E.; Marette, A.; Roy, D.; Desjardins, Y. Berry polyphenols and fibers modulate distinct microbial metabolic functions and gut microbiota enterotype-like clustering in obese mice. Front. Microbiol 2020, 11, 2032. [Google Scholar] [CrossRef] [PubMed]
- Medina-Larque, A.S.; Rodriguez-Daza, M.C.; Roquim, M.; Dudonne, S.; Pilon, G.; Levy, E.; Marette, A.; Roy, D.; Jacques, H.; Desjardins, Y. Cranberry polyphenols and agave agavins impact gut immune response and microbiota composition while improving gut barrier function, inflammation, and glucose metabolism in mice fed an obesogenic diet. Front. Immunol. 2022, 13, 871080. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Martí, À.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, T.M.; Terra, X.; Ardévol, A.; Pinent, M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food Funct. 2018, 9, 1672–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roopchand, D.E.; Kuhn, P.; Rojo, L.E.; Lila, M.; Raskin, I. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacol. Res. 2013, 68, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Carmody, R.N.; Kalariya, H.M.; Duran, R.M.; Moskal, K.; Poulev, A.; Kuhn, P.; Tveter, K.M.; Turnbaugh, P.J.; Raskin, I.; et al. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. J. Nutr. Biochem. 2018, 56, 142–151. [Google Scholar] [CrossRef]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef]
- Anhe, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonne, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Abot, A.; Brochot, A.; Pomie, N.; Wemelle, E.; Druart, C.; Regnier, M.; Delzenne, N.M.; de Vos, W.M.; Knauf, C.; Cani, P.D. Camu-camu reduces obesity and improves diabetic profiles of obese and diabetic mice: A dose-ranging study. Metabolites 2022, 12, 301. [Google Scholar] [CrossRef]
- Xia, T.; Duan, W.; Zhang, Z.; Li, S.; Zhao, Y.; Geng, B.; Zheng, Y.; Yu, J.; Wang, M. Polyphenol-rich vinegar extract regulates intestinal microbiota and immunity and prevents alcohol-induced inflammation in mice. Food Res. Int. 2021, 140, 110064. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Sasaki, G.Y.; Wei, P.; Li, J.; Wang, L.; Zhu, J.; McTigue, D.; Yu, Z.; Bruno, R.S. Green tea extract prevents obesity in male mice by alleviating gut dysbiosis in association with improved intestinal barrier function that limits endotoxin translocation and adipose inflammation. J. Nutr. Biochem. 2019, 67, 78–89. [Google Scholar] [CrossRef]
- Henning, S.M.; Choo, J.J.; Heber, D. Nongallated compared with gallated flavan-3-ols in green and black tea are more bioavailable. J. Nutr. 2008, 138, 1529S–1534S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, M.; Zartl, B.; Schleritzko, A.; Stenzl, M.; Viernstein, H.; Unger, F.M. Rhamnosidase activity of selected probiotics and their ability to hydrolyse flavonoid rhamnoglucosides. Bioprocess. Biosyst. Eng. 2018, 41, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwiazdowska, D.; Jus, K.; Jasnowska-Malecka, J.; Kluczynska, K. The impact of polyphenols on Bifidobacterium growth. Acta Biochim. Pol. 2015, 62, 895–901. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Guo, H.; He, D.; Zhao, H.; Wang, Z.; Zhang, W.; Liao, L.; Zhang, C.; Ni, L. The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice. Food Funct. 2016, 7, 4869–4879. [Google Scholar] [CrossRef]
- Ushiroda, C.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Higashimura, Y.; Yasukawa, Z.; Okubo, T.; Inoue, R.; Honda, A.; et al. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J. Clin. Biochem. Nutr. 2019, 65, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.W.; Kim, J.K.; Kim, A.Y.; Cho, D.; Lee, J.H.; Choi, J.K.; Park, M.; Kim, W. Green tea encourages growth of Akkermansia muciniphila. J. Med. Food 2020, 23, 841–851. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Biotransformation of (-)-epicatechin, (+)-epicatechin, (-)-catechin, and (+)-catechin by intestinal bacteria involved in isoflavone metabolism. Biosci. Biotechnol. Biochem. 2016, 80, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Gaya, P.; Peirotén, Á.; Medina, M.; Álvarez, I.; Landete, J.M. Bifidobacterium pseudocatenulatum INIA P815: The first bacterium able to produce urolithins A and B from ellagic acid. J. Funct. Foods 2018, 45, 95–99. [Google Scholar] [CrossRef]
- Kelly, S.M.; O’Callaghan, J.; Kinsella, M.; van Sinderen, D. Characterisation of a hydroxycinnamic acid esterase from the Bifidobacterium longum subsp. longum taxon. Front. Microbiol. 2018, 9, 2690. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Han, K.; Yang, Q.; Feng, W.; Guo, J.; Wang, J.; Yang, X. Interaction of pyrogallol-containing polyphenols with mucin reinforces intestinal mucus barrier properties. J. Agric. Food Chem. 2022, 70, 9536–9546. [Google Scholar] [CrossRef]
- Al Khalaf, A.K.; Abdulrahman, A.O.; Kaleem, M.; Nur, S.M.; Asseri, A.H.; Choudhry, H.; Khan, M.I. Comparative analysis of the impact of urolithins on the composition of the gut microbiota in normal-diet fed rats. Nutrients 2021, 13, 3885. [Google Scholar] [CrossRef] [PubMed]
- Era, M.; Matsuo, Y.; Saito, Y.; Tanaka, T. Production of.f ellagitannin hexahydroxydiphenoyl ester by spontaneous reduction of dehydrohexa-hydroxydiphenoyl ester. Molecules 2020, 25, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Peng, X.; Li, S.; Zhang, N.; Wang, Y.; Wei, H. Isolation and identification of quercetin degrading bacteria from human fecal microbes. PLoS ONE 2014, 9, e90531. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Rodriguez-Castano, G.P.; Dorris, M.R.; Liu, X.; Bolling, B.W.; Acosta-Gonzalez, A.; Rey, F.E. Bacteroides thetaiotaomicron starch utilization promotes quercetin degradation and butyrate production by Eubacterium ramulus. Front. Microbiol. 2019, 10, 1145. [Google Scholar] [CrossRef]
- Fetzner, S. Ring-Cleaving Dioxygenases with a Cupin Fold. Appl. Environ. Microbiol. 2012, 78, 2505–2514. [Google Scholar] [CrossRef] [Green Version]
- Braune, A.; Gutschow, M.; Engst, W.; Blaut, M. Degradation of quercetin and luteolin by Eubacterium ramulus. Appl. Environ. Microbiol. 2001, 67, 5558–5567. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Zhang, L.; Zhao, G.; Du, X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. Appl. Microbiol. 2019, 127, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food. Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Xu, M.; Qiao, G.; Li, C.; Lin, L.; Zheng, G. Smilax china L. polyphenols alleviates obesity and inflammation by modulating gut microbiota in high fat/high sucrose diet-fed C57BL/6J mice. J. Funct. Foods 2021, 77, 104332. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients 2015, 7, 2788–2800. [Google Scholar] [CrossRef] [Green Version]
- Riva, A.; Kolimar, D.; Spittler, A.; Wisgrill, L.; Herbold, C.W.; Abranko, L.; Berry, D. Conversion of rutin, a prevalent dietary flavonol, by the human gut microbiota. Front. Microbiol. 2020, 11, 585428. [Google Scholar] [CrossRef]
- Bu, F.; Ding, Y.; Chen, T.; Wang, Q.; Wang, R.; Zhou, J.Y.; Jiang, F.; Zhang, D.; Xu, M.; Shi, G.; et al. Total flavone of Abelmoschus Manihot improves colitis by promoting the growth of Akkermansia in mice. Sci. Rep. 2021, 11, 20787. [Google Scholar] [CrossRef]
- Stevens, Y.; Rymenant, E.V.; Grootaert, C.; Camp, J.V.; Possemiers, S.; Masclee, A.; Jonkers, D. The Intestinal fate of citrus flavanones and their effects on gastrointestinal health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef] [Green Version]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escote, X.; Crescenti, A. Effect of hesperidin on cardiovascular disease risk factors: The role of intestinal microbiota on hesperidin bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, T.; Lei, C.; Song, W.; Fang, R.; Chen, H.; Li, C.; Li, X.; Liang, X.; Huang, Q. Novel role of hesperidin improve obesity in HFD mice by modulating the composition of the gut microbiota. Res. Sq. 2020, preprint. [Google Scholar] [CrossRef]
- Blaut, M.; Schoefer, L.; Braune, A. Transformation of flavonoids by intestinal microorganisms. Int. J. Vitam. Nutr. Res. 2003, 73, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Schoefer, L.; Braune, A.; Blaut, M. Cloning and expression of a phloretin hydrolase gene from Eubacterium ramulus and characterization of the recombinant enzyme. Appl. Environ. Microbiol. 2004, 70, 6131–6137. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Firrman, J.; Zhang, L.; Arango-Argoty, G.; Tomasula, P.; Liu, L.; Xiao, W.; Yam, K. Apigenin impacts the growth of the gut microbiota and alters the gene expression of Enterococcus. Molecules 2017, 22, 1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Zhang, Z.; Zhai, Y.; Yan, X.; Zhou, W.; Liu, H.; Guan, L.; Peng, L. Apigenin alleviates obesity-associated metabolic syndrome by regulating the composition of the gut microbiome. Front. Microbiol. 2021, 12, 805827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Troise, A.D.; Qi, Y.; Wu, G.; Zhang, H.; Fogliano, V. Insoluble dietary fibre scavenges reactive carbonyl species under simulated physiological conditions: The key role of fibre-bound polyphenols. Food Chem. 2021, 349, 129018. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Dionisi, F.; Renouf, M. Flavanols from green tea and phenolic acids from coffee: Critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol. Nutr. Food Res. 2011, 55, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wu, X.; Cao, S.; Cromie, M.; Shen, Y.; Feng, Y.; Yang, H.; Li, L. Chlorogenic acid ameliorates experimental colitis by promoting growth of Akkermansia in mice. Nutrients 2017, 9, 677. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wu, X.; Cao, S.; Wang, L.; Wang, D.; Yang, H.; Feng, Y.; Wang, S.; Li, L. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget 2016, 7, 31790–31799. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, D.; Qiao, S.; Wu, X.; Cao, S.; Wang, L.; Su, X.; Li, L. Metabolic and microbial signatures in rat hepatocellular carcinoma treated with caffeic acid and chlorogenic acid. Sci. Rep. 2017, 7, 4508. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, M.; Dong, L.; Jia, X.; Liu, L.; Ma, Y.; Huang, F.; Zhang, R. Phytochemical profile, bioactivity, and prebiotic potential of bound phenolics released from rice bran dietary fiber during in vitro gastrointestinal digestion and colonic fermentation. J. Agric. Food Chem. 2019, 67, 12796–12805. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, C.; Jansch, A.; Ehrmann, M.A.; Toelstede, S.; Vogel, R.F. Characterization of cinnamoyl esterases from different Lactobacilli and Bifidobacteria. Curr. Microbiol. 2017, 74, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Torres, M.; Landete, J.M.; Reveron, I.; Santamaria, L.; de las Rivas, B.; Munoz, R. A Lactobacillus plantarum esterase active on a broad range of phenolic esters. Appl. Environ. Microbiol. 2015, 81, 3235–3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaria, L.; Reveron, I.; Lopez de Felipe, F.; de Las Rivas, B.; Munoz, R. Unravelling the reduction pathway as an alternative metabolic route to hydroxycinnamate decarboxylation in Lactobacillus plantarum. Appl. Environ. Microbiol. 2018, 84, e01123-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poti, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: A review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias-Aguirre, C.E.; Vallejo, F.; Beltran, D.; Berna, J.; Puigcerver, J.; Alajarin, M.; Selma, M.V.; Espin, J.C. 4-Hydroxydibenzyl: A novel metabolite from the human gut microbiota after consuming resveratrol. Food Funct 2022, 13, 7487–7493. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Hou, P.; Zhou, M.; Ren, Q.; Wang, X.; Huang, L.; Hui, S.; Yi, L.; Mi, M. Resveratrol attenuates high-fat diet-induced non-alcoholic steatohepatitis by maintaining gut barrier integrity and inhibiting gut inflammation through regulation of the endocannabinoid system. Clin. Nutr. 2020, 39, 1264–1275. [Google Scholar] [CrossRef]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc. Biol. 2019, 106, 467–480. [Google Scholar] [CrossRef]
- Walker, J.M.; Eckardt, P.; Aleman, J.O.; Correa da Rosa, J.; Liang, Y.; Lizumi, T.; Etheve, S.; Blaser, M.J.; Breslow, J.L.; Holt, P.R. The effects of trans-resveratrol on insulin resistance, inflammation, and microbiota in men with the metabolic syndrome: A pilot randomized, placebo controlled clinical trial. J. Clin. Transl. Res. 2018, 4, 122–135. [Google Scholar] [PubMed]
- Etxeberria, U.; Hijona, E.; Aguirre, L.; Milagro, F.I.; Bujanda, L.; Rimando, A.M.; Martinez, J.A.; Portillo, M.P. Pterostilbene-induced changes in gut microbiota composition in relation to obesity. Mol. Nutr. Food Res. 2017, 61, 1500906. [Google Scholar] [CrossRef] [PubMed]
- Kala, R.; Tollefsbol, T.O.; Li, Y. Potential of resveratrol in inhibiting cancer and slowing aging. J. Nutr. Sci. 2012, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Quartieri, A.; Garcia-Villalba, R.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M.; Tomas-Barberan, F. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol. Nutr. Food Res. 2016, 60, 1590–1601. [Google Scholar] [CrossRef]
- Clavel, T.; Borrmann, D.; Braune, A.; Dore, J.; Blaut, M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe 2006, 12, 140–147. [Google Scholar] [CrossRef]
- Mabrok, H.B.; Klopfleisch, R.; Ghanem, K.Z.; Clavel, T.; Blaut, M.; Loh, G. Lignan transformation by gut bacteria lowers tumor burden in a gnotobiotic rat model of breast cancer. Carcinogenesis 2012, 33, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Power, K.A.; Lepp, D.; Zarepoor, L.; Monk, J.M.; Wu, W.; Tsao, R.; Liu, R. Dietary flaxseed modulates the colonic microenvironment in healthy C57Bl/6 male mice which may alter susceptibility to gut-associated diseases. J. Nutr. Biochem. 2016, 28, 61–69. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, J.; Lee, J.H.; Sim, J.H.; Cho, D.H.; Bae, I.H.; Lee, H.; Seol, M.A.; Shin, H.M.; Kim, T.J.; et al. Modulation of gut microbiota and delayed immunosenescence as a result of syringaresinol consumption in middle-aged mice. Sci. Rep. 2016, 6, 39026. [Google Scholar] [CrossRef] [Green Version]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Korsholm, A.S.; Kjaer, T.N.; Ornstrup, M.J.; Pedersen, S.B. Comprehensive metabolomic analysis in blood, urine, fat, and muscle in men with metabolic syndrome: A randomized, placebo-controlled clinical trial on the effects of resveratrol after four months’ treatment. Int. J. Mol. Sci. 2017, 18, 544. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Feng, M.; Chu, Y.; Wang, S.; Shete, V.; Tuohy, K.M.; Liu, F.; Zhou, X.; Kamil, A.; Pan, D.; et al. The prebiotic effects of oats on blood lipids, gut microbiota, and short-chain fatty acids in mildly hypercholesterolemic subjects compared with rice: A randomized, controlled trial. Front. Immunol. 2021, 12, 787797. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Penders, J.; Lucchesi, M.; Goossens, G.H.; Blaak, E.E. Gut microbiota composition in relation to the metabolic response to 12-week combined polyphenol supplementation in overweight men and women. Eur. J. Clin. Nutr. 2017, 71, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-y.; Wang, J.-h.; Eom, T.; Kim, H. Schisandra chinensis fruit modulates the gut microbiota composition in association with metabolic markers in obese women: A randomized, double-blind placebo-controlled study. Nutr. Res. 2015, 35, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Venancio, V.P.; Fang, C.; Dupont, A.W.; Talcott, S.T.; Mertens-Talcott, S.U. Mango (Mangifera indica L.) polyphenols reduce IL-8, GRO, and GM-SCF plasma levels and increase Lactobacillus species in a pilot study in patients with inflammatory bowel disease. Nutr. Res. 2020, 75, 85–94. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food. Res. 2017, 61, 1500901. [Google Scholar] [CrossRef]
- Atkinson, C.; Newton, K.M.; Bowles, E.J.; Yong, M.; Lampe, J.W. Demographic, anthropometric, and lifestyle factors and dietary intakes in relation to daidzein-metabolizing phenotypes among premenopausal women in the United States. Am. J. Clin. Nutr. 2008, 87, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Selma, M.V.; Beltran, D.; Garcia-Villalba, R.; Espin, J.C.; Tomas-Barberan, F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef] [Green Version]
- Cortes-Martin, A.; Romo-Vaquero, M.; Garcia-Mantrana, I.; Rodriguez-Varela, A.; Collado, M.C.; Espin, J.C.; Selma, M.V. Urolithin metabotypes can anticipate the different restoration of the gut microbiota and anthropometric profiles during the first year postpartum. Nutrients 2019, 11, 2079. [Google Scholar] [CrossRef] [Green Version]
- Tomas-Barberan, F.A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Selma, M.V.; Espin, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Polyphenols’ gut microbiota metabolites: Bioactives or biomarkers? J. Agric. Food Chem. 2018, 66, 3593–3594. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Martin, A.; Garcia-Villalba, R.; Garcia-Mantrana, I.; Rodriguez-Varela, A.; Romo-Vaquero, M.; Collado, M.C.; Tomas-Barberan, F.A.; Espin, J.C.; Selma, M.V. Urolithins in human breast milk after walnut intake and kinetics of Gordonibacter colonization in newly born: The role of mothers’ urolithin metabotypes. J. Agric. Food Chem. 2020, 68, 12606–12616. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Watanabe, K.; Kimura, I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front. Immunol. 2017, 8, 1882. [Google Scholar] [CrossRef] [PubMed]
- Van Herreweghen, F.; De Paepe, K.; Marzorati, M.; Van de Wiele, T. Mucin as a functional niche is a more important driver of in vitro gut microbiota composition and functionality than supplementation of Akkermansia muciniphila. Appl Environ. Microbiol. 2020, 87, e02647-20. [Google Scholar] [CrossRef]
- Liu, F.; Cui, Y.; Yang, F.; Xu, Z.; Da, L.T.; Zhang, Y. Inhibition of polypeptide N-acetyl-alpha-galactosaminyltransferases is an underlying mechanism of dietary polyphenols preventing colorectal tumorigenesis. Bioorg. Med. Chem. 2019, 27, 3372–3382. [Google Scholar] [CrossRef]
- Gabrielli, M.G.; Tomassoni, D. Starch-enriched diet modulates the glucidic profile in the rat colonic mucosa. Eur. J. Nutr. 2018, 57, 1109–1121. [Google Scholar] [CrossRef]
- Lu, F.; Li, Y.; Wang, X.; Hu, X.; Liao, X.; Zhang, Y. Early-life polyphenol intake promotes Akkermansia growth and increase of host goblet cells in association with the potential synergistic effect of Lactobacillus. Food Res. Int. 2021, 149, 110648. [Google Scholar] [CrossRef]
- Alvarado, D.M.; Chen, B.; Iticovici, M.; Thaker, A.I.; Dai, N.; VanDussen, K.L.; Shaikh, N.; Lim, C.K.; Guillemin, G.J.; Tarr, P.I.; et al. Epithelial indoleamine 2,3-dioxygenase 1 modulates aryl hydrocarbon receptor and notch signaling to increase differentiation of secretory cells and alter mucus-associated microbiota. Gastroenterology 2019, 157, 1093–1108.e11. [Google Scholar] [CrossRef]
- Powell, D.N.; Swimm, A.; Sonowal, R.; Bretin, A.; Gewirtz, A.T.; Jones, R.M.; Kalman, D. Indoles from the commensal microbiota act via the AHR and IL-10 to tune the cellular composition of the colonic epithelium during aging. Proc. Natl. Acad. Sci. USA 2020, 117, 21519–21526. [Google Scholar] [CrossRef]
- Stockinger, B.; Shah, K.; Wincent, E. AHR in the intestinal microenvironment: Safeguarding barrier function. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 559–570. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Tian, H.; Tian, F.; Zhang, Y.; Zhang, L.; Gao, X.; Wang, X. Aryl hydrocarbon receptor/IL-22/Stat3 signaling pathway is involved in the modulation of intestinal mucosa antimicrobial molecules by commensal microbiota in mice. Innate Immun. 2018, 24, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Goya-Jorge, E.; Jorge Rodriguez, M.E.; Veitia, M.S.; Giner, R.M. Plant occurring flavonoids as modulators of the aryl hydrocarbon receptor. Molecules 2021, 26, 2315. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, L.; Ciocan, D.; Hugot, C.; Spatz, M.; Dupeux, M.; Houron, C.; Lievin-Le Moal, V.; Puchois, V.; Ferrere, G.; Trainel, N.; et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut 2021, 70, 1299–1308. [Google Scholar] [CrossRef]
- Carambia, A.; Schuran, F.A. The aryl hydrocarbon receptor in liver inflammation. Semin. Immunopathol. 2021, 43, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Borucki, D.M.; Kenison, J.E.; Hewson, P.; Wang, Z.; Bakshi, R.; Sherr, D.H.; Quintana, F.J. Detection of aryl hydrocarbon receptor agonists in human samples. Sci. Rep. 2018, 8, 4970. [Google Scholar] [CrossRef] [Green Version]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 2018, 28, 737–749.e4. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Lei, H.; Chen, G.; Yuan, P.; Cao, Z.; Ser, H.L.; Zhu, X.; Wu, F.; Liu, C.; Dong, M.; et al. Impaired Intestinal Akkermansia muciniphila and aryl hydrocarbon receptor ligands contribute to nonalcoholic fatty liver disease in mice. mSystems 2021, 6, e00985-20. [Google Scholar] [CrossRef]
- Jin, U.H.; Park, H.; Li, X.; Davidson, L.A.; Allred, C.; Patil, B.; Jayaprakasha, G.; Orr, A.A.; Mao, L.; Chapkin, R.S.; et al. Structure-dependent modulation of aryl hydrocarbon receptor-mediated activities by flavonoids. Toxicol. Sci. 2018, 164, 205–217. [Google Scholar] [CrossRef] [Green Version]
- Koper, J.E.B.; Loonen, L.M.P.; Wells, J.M.; Troise, A.D.; Capuano, E.; Fogliano, V. Polyphenols and tryptophan metabolites activate the aryl hydrocarbon receptor in an in vitro model of colonic fermentation. Mol. Nutr. Food Res. 2019, 63, e1800722. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef] [PubMed]
- Rannug, A. How the AhR became important in intestinal homeostasis- A Diurnal FICZ/AhR/CYP1A1 feedback controls both Iimunity and immunopathology. Int. J. Mol. Sci. 2020, 21, 5681. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011, 141, 237–248.e1. [Google Scholar] [CrossRef] [PubMed]
- Meynier, M.; Baudu, E.; Rolhion, N.; Defaye, M.; Straube, M.; Daugey, V.; Modoux, M.; Wawrzyniak, I.; Delbac, F.; Villeger, R.; et al. AhR/IL-22 pathway as new target for the treatment of post-infectious irritable bowel syndrome symptoms. Gut Microbes 2022, 14, 2022997. [Google Scholar] [CrossRef]
- Han, H.; Davidson, L.A.; Fan, Y.Y.; Landrock, K.K.; Jayaraman, A.; Safe, S.H.; Chapkin, R.S. Loss of aryl hydrocarbon receptor suppresses the response of colonic epithelial cells to IL22 signaling by upregulating SOCS3. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G93–G106. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.; Spalinger, M.R.; Gottier, C.; Biedermann, L.; Zeitz, J.; Lang, S.; Weber, A.; Rogler, G.; Scharl, M. Bilberry-derived anthocyanins modulate cytokine expression in the intestine of patients with ulcerative colitis. PLoS ONE 2016, 11, e0154817. [Google Scholar] [CrossRef] [Green Version]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Tabasco, R.; Sánchez-Patán, F.; Monagas, M.; Bartolomé, B.; Moreno-Arribas, V.M.; Peláez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011, 28, 1345–1352. [Google Scholar] [CrossRef]
- Dong, J.; Qiu, J.; Wang, J.; Li, H.; Dai, X.; Zhang, Y.; Wang, X.; Tan, W.; Niu, X.; Deng, X.; et al. Apigenin alleviates the symptoms of Staphylococcus aureus pneumonia by inhibiting the production of alpha-hemolysin. FEMS Microbiol. Lett. 2013, 338, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Lopez de Felipe, F.; de Las Rivas, B.; Munoz, R. Molecular responses of Lactobacilli to plant phenolic compounds: A comparative review of the mechanisms involved. Antioxidants 2021, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yi, Z.B.; Liang, Y.Z. Validate antibacterial mode and find main bioactive components of traditional Chinese medicine Aquilegia oxysepala. Bioorg. Med. Chem. Lett. 2007, 17, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine:D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Martinez, F.J.; Barrajon-Catalan, E.; Encinar, J.A.; Rodriguez-Diaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against Gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef]
- Hansen, C.H.F.; Krych, L.; Nielsen, D.S.; Diabetologia, V.-F.K. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 2012, 55, 2285–2294. [Google Scholar] [CrossRef] [Green Version]
- Dubourg, G.; Lagier, J.-C.; Armougom, F.; Robert, C.; Audoly, G.; Papazian, L.; Raoult, D. High-level colonisation of the human gut by Verrucomicrobia following broad-spectrum antibiotic treatment. Int. J. Antimicrob. Agents 2013, 41, 149–155. [Google Scholar] [CrossRef]
- Hagi, T.; Geerlings, S.Y.; Nijsse, B.; Belzer, C. The effect of bile acids on the growth and global gene expression profiles in Akkermansia muciniphila. Appl. Microbiol. Biotechnol. 2020, 104, 10641–10653. [Google Scholar] [CrossRef]
- Shin, J.; Noh, J.-R.; Chang, D.-H.; Kim, Y.-H.; Kim, M.; Lee, E.; Cho, S.; Ku, B.; Rhee, M.-S.; Kim, B.-C.; et al. Elucidation of Akkermansia muciniphila probiotic traits driven by mucin depletion. Front. Microbiol. 2019, 10, 1137. [Google Scholar] [CrossRef] [Green Version]
- Das, Q.; Lepp, D.; Yin, X.; Ross, K.; McCallum, J.L.; Warriner, K.; Marcone, M.F.; Diarra, M.S. Transcriptional profiling of Salmonella enterica serovar Enteritidis exposed to ethanolic extract of organic cranberry pomace. PLoS ONE 2019, 14, e0219163. [Google Scholar] [CrossRef] [Green Version]
- Curiel, J.A.; Rodríguez, H.; De la Rivas, B.; Anglade, O.; Baraige, F.; Zagorec, M.; Champomier-Vergès, M.; Muñoz, R.; de Felipe, F. Response of a Lactobacillus plantarum human isolate to tannic acid challenge assessed by proteomic analyses. Mol. Nutr. Food Res. 2011, 55, 1454–1465. [Google Scholar] [CrossRef]
- Reveron, I.; de las Rivas, B.; Matesanz, R.; Munoz, R.; Lopez de Felipe, F. Molecular adaptation of Lactobacillus plantarum WCFS1 to gallic acid revealed by genome-scale transcriptomic signature and physiological analysis. Microb. Cell Fact. 2015, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.K.S. Influence of gallic acid and catechin polyphenols on probiotic properties of Streptococcus thermophilus CHCC 3534 strain. World J. Microbiol. Biotechnol. 2010, 26, 2069–2079. [Google Scholar] [CrossRef]
- Firrman, J.; Liu, L.; Zhang, L.; Argoty, G.; Wang, M.; Tomasula, P.; Kobori, M.; Pontious, S.; Xiao, W. The effect of quercetin on genetic expression of the commensal gut microbes Bifidobacterium catenulatum, Enterococcus caccae and Ruminococcus gauvreauii. Anaerobe 2016, 42, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Schiller, N.L.; Kahng, H.Y.; Oh, K.H. Cellular responses and proteomic analysis of Escherichia coli exposed to green tea polyphenols. Curr. Microbiol. 2007, 55, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Simmering, R.; Pforte, H.; Jacobasch, G.; Blaut, M. The growth of the flavonoid-degrading intestinal bacterium, Eubacterium ramulus, is stimulated by dietary flavonoids in vivo. FEMS Microbiol. Ecol. 2002, 40, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Kathagen, G.; D’Hoe, K.; Vieira-Silva, S.; Valles-Colomer, M.; Sabino, J.; Wang, J.; Tito, R.Y.; De Commer, L.; Darzi, Y.; et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 2017, 551, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.P.; Martelli-Tosi, M.; Massarioli, A.P.; Melo, P.S.; Alencar, S.M.; Favaro-Trindade, C.S. Co-encapsulation of guaraná extracts and probiotics increases probiotic survivability and simultaneously delivers bioactive compounds in simulated gastrointestinal fluids. LWT 2022, 161, 113351. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, Y.; Xu, N.; Mu, H.; Zhang, H.; Duan, J. Improved viability of Akkermansia muciniphila by encapsulation in spray dried succinate-grafted alginate doped with epigallocatechin-3-gallate. Int. J. Biol. Macromol. 2020, 159, 373–382. [Google Scholar] [CrossRef]
- Kvakova, M.; Bertkova, I.; Stofilova, J.; Savidge, T.C. Co-encapsulated synbiotics and immobilized probiotics in human health and gut microbiota modulation. Foods 2021, 10, 1297. [Google Scholar] [CrossRef]




| Polyphenol-Rich Food | Experimental Design | Main Findings in the Gut Microbiota | A. muciniphila Modulation | Impact on Host Health | Ref. | |
|---|---|---|---|---|---|---|
| Anthocyanins | Açai extract | SPF C57BL/6J mice fed a HFD for 14 weeks with daily gavage of 150 mg/kg of anthocyanin-rich açai extract | ↑ Parabacteroides distasonis and B. acidifaciens. | ↑ A. muciniphila was significantly and negatively associated with serum TG, glucose, and insulin | Enhanced liver damage, glucose intolerance, and insulin resistance | [86] |
| Davidson’s plum | HCHF-induced obesity in Wistar rats supplemented with 8 mg of anthocyanins equivalent/kg/day for 8 weeks | ↓ Clostridiaceae, ↑ Turicibacter spp. | ↑ A. muciniphila | Reduction in visceral fat accumulation, inflammation, and plasma TG | [87] | |
| Bilberry | Western diet (WD)-induced NAFLD in male C57BL/6N mice for 18 weeks supplemented with 2% bilberry anthocyanins | ↓ F/B, Prevotella spp., Lactobacillales, and Clostridiales; ↑B. acidifaciens; ↑Parabacteroides spp. | ↑ A. muciniphila was negatively correlated with liver injury | Attenuated liver injury and dyslipidemia | [37] | |
| Black raspberries (BRBs) | 5% whole BRB powder, 0.2% BRB anthocyanins or 2.25% of the residue fraction supplemented in F-344 rats under a standard diet for 6 weeks | 5% whole BRB powder; ↑ Anaerostipes; ↑ Ruminococcus; ↑ Coprobacillus; ↓ Acetivibrio; ↓ Anaerotruncus spp. | ↑ A. muciniphila by the whole BRB and the residue | Enhanced inflammatory biomarkers | [88] | |
| Polyphenol-Rich Foods | Experimental Design | Main Findings in the Gut Microbiota | A. muciniphila Modulation | Impact on Host Health | Ref. | |
|---|---|---|---|---|---|---|
| Flavan-3-ols | Wild blueberry and PACs fractions | HFHS-induced obesity in male C57BL/6N mice gavaged daily with 200 mg/kg BW of WBE or equivalent PACs for 8 weeks | ↑2-fold Adlercreutzia equolifaciens* (Coriobacteriaceae) by the WBE and PACs | ↑2.5-fold A. muciniphila (24.8%) | Enhanced glucose tolerance, ↑GC, restored colon mucus layer | [100] |
| Cranberry juice (57% PACs) | Germ-free C57BL/6 with a simplified human microbiome gavaged daily with 200 mg/kg BW | ↑Bacteroides ovatus, ↑Clostridium hiranonis | ↑A. muciniphila | ↑Intestinal mucus accumulation | [101] | |
| Cranberry (CP) and blueberry (BP) extracts (PACs) and their fibrous residues (CF and BF) | HFHS-induced obesity in male C57BL/6N mice fed 200 mg/kg berry powder or the equivalent fibrous fractions for 8 weeks | ↓F/B, ↑Dubosiella newyorkensis, ↑Angelakisella spp., ↑Coriobacteriaceae* ↑Eggerthellaceae,*. ↓Lachnospiraceae, ↓Ruminococcaceae, ↓Peptostreptococcaceae | ↑A. muciniphila by CP and BP correlated with lower BW | ↓Fat mass depots, ↓BW, ↑mucus layer thickness | [102] | |
| Peach peel extract (PPE) (28% epicatechin 3-O-glucoside and 12.7% PACs) | HFD-induced obesity in female ICR mice fed 300 mg/kg (HPP) or 150 mg/kg (LPP) BW for 12 weeks | ↓F/B. ↑Lactobacillus spp.,* ↑Bifidobacterium spp.,* ↑Roseburia spp., ↑Bacteroides spp.,* ↑Lachnospiraceae,* ↑Prevotellaceae, and ↑Alloprevotella spp. | ↑A. muciniphila by HPP and LPP | ↓BW, ↓oxidative stress, ↓hepatic lipid accumulation, ↑butyrate | [96] | |
| Cranberry extract (CP) (PACs) and agave inulin (AG) | HFHS-induced obesity in male C57BL/6N mice gavaged with either 200 mg/kg BW CP, 1 g/kg BW AG, or both for 9 weeks | ↑Muribaculum spp., ↑Faecalibaculum rodentium, ↑Roseburia spp., ↑Alistipes spp. ↑Bacteroidaceae*, ↓Ruminiclostridium spp., ↓Lachnospiraceae, ↓Peptococcaceae | ↑5.0-fold A. muciniphila only by CP | ↑GC number, ↑Nlrp6, improved glucose tolerance, ↑TLR2, ↑AhR, ↓colon inflammation | [103] | |
| Polyphenol-Rich Foods | Experimental Design | Main Findings in the Gut Microbiota | A. muciniphila Modulation | Impact on Host Health | Ref. | |
|---|---|---|---|---|---|---|
| Flavan-3-ols | Apple polymeric proanthocyanins (PACs) | C57BL/6J mice fed an HFHS diet for 20 weeks supplemented with 0.5% polymeric PACs | ↓F/B., ↑Adlercreutzia spp.,* ↑Roseburia spp., ↑S24-7, ↑Bacteroides spp., ↓Clostridium, ↓Lachnospiraceae, ↓Bifidobacterium spp.* | ↑8.0-fold A. muciniphila | ↓Dyslipidemia, ↓liver damage, ↓insulin resistance; ↓inflammation, ↓intestinal permeability | [107] |
| Grape polyphenols (GP) (catechins) | HFD-induced obesity in male C57BL/6J mice fed 1% GP for 13 weeks | ↓F/B, ↑Alistipes spp., ↑Raouterella spp. ↓Lactobacillus spp., ↓Turicibacter spp., ↓Lachnospiraceae, ↓Clostridiales | ↑A. muciniphila 49% and 54.8% in cecum and feces, respectively | ↓BW gain, ↓adiposity, endotoxemia, and improved glucose intolerance | [111] | |
| Camu-camu(CC) (galloylated PACs and ellagitannins) | HFHS-induced obesity in male C57Bl/6J fed 200 mg/kg CC for 8 weeks | ↓Lactobacillus spp., ↑Barnesiella spp., ↑Bifidobacterium spp., ↑Turicibacter spp. | ↑A. muciniphila and correlated with plasma bile acids | ↓Hepatosteatosis, ↓metabolic endotoxemia, ↓glucose intolerance | [108] | |
| Camu-camu polyphenolic extract (CCE) | HFD-induced obesity in male C57BL/6J mice fed 200 mg/kg or 62.5 mg/kg CCE for 5 weeks | NA | ↑A. muciniphila by 62.5 mg/kg CCE | ↓Dyslipidemia, ↓BW, ↓hepatosteatosis | [109] | |
| Catechin-rich Zhenjiang aromatic vinegar extract (ZAVE) | Long-term alcohol consumption in ICR male mice gavaged daily with 200 or 800 mg/kg BW of ZAVE for 30 days | ↓F/B, ↑Lachnospiraceae_NK4A136_group, ↑Bacteroides spp., ↓Bilophila and ↓Butyricimonas spp. | ↑A. muciniphila, correlated with ↑antimicrobial peptides, ↓oxidative stress, and ↓inflammation | ↓Gut inflammation, ↑IL-22, ↑Reg3g, ↓liver damage | [110] | |
| Green tea (GTE) (48% EGCG) | HFD-induced obesity in male C57BL/6J mice fed 2% GTE for 8 weeks | ↓F/B, ↑microbial diversity, ↑Actinobacteria, ↑Coriobacteriales, ↑Turicibacterales, ↓Clostridiales, ↑B. pseudolongum*, ↑B. adolescentis* | ↑A. muciniphila by all the tea infusions | ↓Adipose inflammation, ↓metabolic endotoxemia, ↑TJPs in ileum and colon | [112] | |
| Green, oolong, and black teas (flavan-3-ols) | Male C57BL/6J mice fed an HFD supplemented with 45% energy from fat as food and tea infusions as drinking water for 13 weeks | ↑Microbial diversity, ↑Alistipes, ↑Lachnospiraceae, ↑Rikenella microfusus, ↓Allobaculum spp., ↓B. acidifaciens, ↓Clostridium leptum, ↓Parabacteroides goldsteinii | ↑A. muciniphila and negatively correlated with serum LBP levels | ↓BW, ↓fat tissue accumulation, ↓metabolic endotoxemia, ↑lipid and glucose metabolism | [113] | |
| Phenolic Metabolites | Urolithin A (UA) and urolithin B (UB) | Male Wistar rats fed a normal diet receiving an IP injection of UA or UB (2.5 mg/kg each) for 4 weeks | ↓F/B, ↑Flavobacteriales by UA, ↓Lactobacillales and, ↓Clostridiales by UA and UB | ↑A. muciniphilia by UA and UB | ↓Serum AST | [114] |
| Polyphenol-Rich Foods | Experimental Design | Main Findings in the Gut Microbiota | A. muciniphila Modulation | Impact on Host Health | Ref. | |
|---|---|---|---|---|---|---|
| Flavonols | Quercetin | HFD-induced obesity in LDLR−/− (LDL receptor-deficient) C57BL/6 mice fed 100 μg of quercetin daily for 12 weeks | ↑Microbial diversity, ↑Actinobacteria, ↑Bacteroidota ↓Firmicutes, ↓Lactobacillus,* ↑Bacteroides,* ↑Parabacteroides, ↑Ruminococcus | ↑2.0-fold A. muciniphila | ↓BW, ↓Intestinal cholesterol, ↓oxidative stress, ↓inflammation | [131] |
| HFHS-induced obesity in Wistar rats fed 30 mg/kg BW quercetin for 6 weeks | ↓F/B, ↓Erysipelotrichaceae, ↓Bacillus, ↓Eubacterium cylindroides, ↑Barnesiella, ↑Bacteroides dorei, ↑Bacteroides chinchillae, ↑Prevotella | ↑1-8-fold A. muciniphila (13.84%) | ↓Insulin resistance, ↑TJPs | [132] | ||
| Quercetin + resveratrol | HFD-induced obesity in Wistar rats fed 30 mg/kg BW quercetin, 15 mg/kg BW of resveratrol, or both for 10 weeks | ↑Microbial diversity, ↑Bacteroidales_S24-7_group, ↑Ruminococca-ceae, ↑Christensenellaceae, ↓Lachnoclostridium, ↓Bilophila | ↑ A. muciniphila | ↓BW, ↓serum lipids, ↓inflammatory markers | [133] | |
| Quercetin (Q) + A. muciniphila cells | HFD-induced obesity in Wistar rats fed with 2 × 108 CFU/200 µL and 37.5 mg/kg Q for 3 weeks | ↑Cyanobacteria, ↑Oscillospira spp., ↓Actinobacteria, ↓Lactococcus spp., ↓Lactobacillus spp.,* ↓Blautia spp., ↓Rothia spp., ↓Roseburia spp. | ↑A. muciniphila only when co-administered with Q. It correlated with ↓BW, lipid, and bile acid metabolism | ↓BW, ↓fat mass depot, ↓NAFLD | [39] | |
| Abelmoschus manihot flowers (TFA) (Flavonol glycosides) | DSS-induced colitis in C57BL/6J mice gavaged with 125 mg/kg or 62.5 mg/kg of TFA for 7 days | ↑Gordonibacter spp.,* ↑Erysipelatoclostridium spp., ↓Tenericutes, ↓Proteobacteria | ↑A. muciniphila (only by 125 mg/kg TFA), correlated with ↓gut inflammation, ↑Muc2, ↑barrier function | ↓ Colonic inflammatory, ↓intestinal epithelial barrier dysfunction | [137] | |
| Polyphenol-Rich Foods | Experimental Design | Main Findings in the Gut Microbiota | A. muciniphila Modulation | Impact on Host Health | Ref. | |
|---|---|---|---|---|---|---|
| Flavanones | Hesperidin | HFD-induced obesity in male C57BL/6 mice gavaged with 100 or 200 mg/kg BW hesperidin for 10 weeks | ↑Lactobacillus salivarius, ↑Desulfovibrio _C21_c20, ↓Helicobacter spp., ↓B. pseudolongum and ↓Mucispirillum schaedleri | Failed to change A. muciniphila | ↓BW, ↓inflammation, ↓plasma LBP, ↑intestinal integrity | [140] |
| Flavonones | Apigenin | HFD-induced obese male C57BL/6 J mice gavaged with 50 mg/kg BW apigenin for 16 weeks | ↓F/B, ↑Bacteroidaceae, ↓Erysipelotrichaceae | ↑A. muciniphila (Akkermansiaceae) | ↓Metabolic endotoxemia, ↓inflammation, ↓liver injury, ↓hepatosteatosis, ↑intestinal integrity | [144] |
| Phenolic acids | Caffeic acid (CaA) | DSS-induced colitis in female C57BL/6 mice gavaged with 1 mM CaA for 15 days | ↑Microbial diversity, ↓F/B, ↑Tenericutes | ↑A. muciniphila (25%) | ↓Gut and serum inflammatory markers, ↓NF-κB signaling pathways | [149] |
| Chlorogenic acid (ChA) | DSS-induced colitis in female C57BL/6 mice gavaged with 1 mM ChA for 15 days | ↓F/B, ↑microbial diversity | ↑A. muciniphila (38%) | ↓Diarrhea and rectal bleeding, ↓mucin depletion, ↓gut inflammation | [148] | |
| Rice bran fiber-bound phenolic acids (RBDF) (p-coumaric acid, hydroxybenzoic acid, and ferulic acid) | In vitro colonic fermentation of GI-digested RBDF | ↑F. prausnitzii, ↑Bifidobacterium,* ↑Lactobacillus* spp. | ↑A. muciniphila only by the fiber-bound phenolics but not by the phenolic-free fibers | ↑Antioxidant and hypoglycemic activities | [151] | |
| Polyphenol-Rich Foods | Experimental Design | Main Findings in the Gut Microbiota | A. Muciniphila Modulation | Impact on Host Health | Ref. | |
|---|---|---|---|---|---|---|
| Stilbenes | Resveratrol (RSV) | TMAO-induced atherosclerosis in ApoE−/− female C57BL/6 mice fed a chow diet with 0.4%RSV for 30 days | ↑Bacteroides,* ↑Lactobacillus spp.,* ↑Bifidobacterium spp.,* ↓Prevotella spp., ↓Ruminococcaceae, ↓Anaerotruncus spp., ↓Alistipes spp., ↓Peptococcaceae | ↑A. muciniphila | Protected against atherosclerosis, ↓gut microbial TMA production | [158] |
| HFD-induced obesity in male Sprague–Dawley rats fed 100 mg/kg RSV for 6 weeks | ↑Ruminococcaceae, ↑Lachnospiraceae, ↓Desulfovibrio spp. | ↑A. muciniphila, correlated with endocannabinoid system modulation. | ↑TJPs, ↓CB1, ↓CB2, ↓steatohepatitis, ↓gut inflammation, ↓metabolic endotoxemia | [159] | ||
| TNBS-induced colitis in female BALB/c mice fed 100 mg/kg RSV for 5 days | ↓B. acidifaciens, ↑Ruminococcus gnavus | ↑4.5-fold A. muciniphila | Attenuated colitis, ↓gut inflammation, ↑butyrate | [160] | ||
| Pterostilbene (Pst) | Zucker (fa/fa) rats fed a standard diet and gavaged with 15 mg/kg BW Pst for 6 weeks | ↓F/B, ↑Odoribacter splanchnicus | ↑A. muciniphila, correlated with ↓BW | ↓BW, ↓fat mass, ↓serum insulin, ↓glucose intolerance | [162] | |
| Lignans | Flaxseed (FS) (secoisolariciresinol diglucoside) | C57BL/6 mice fed a standard diet with 10% FS for 3 weeks | ↑20-fold Prevotella spp., ↑10-fold Roseburia spp. | ↓30-fold A. muciniphila | ↑GC, ↑Muc2, ↑RegIIIγ in the colon | [167] |
| Syringaresinol (SYR) | Middle-aged male C57BL/6 mice fed 10 or 50 mg/kg BW of SYR for 10 weeks | ↓F/B, ↑Lactobacillus spp.,* ↑B. pseudolongum,* ↓Bacteroidaceae* | ↓A. muciniphila by high dose of SYR | ↓LBP, ↑Foxp3+ regulatory T cells | [168] | |
| Polyphenol-Rich Foods | Experimental Design | Main Findings in the Gut Microbiota | A. muciniphila Modulation | Impact on Host Health | Ref. | |
|---|---|---|---|---|---|---|
| Phenolic acids | Oats rich in (β-glucans and polyphenol s) | Intake of 80 g of oat comprising by β-glucans (3.0 g) and polyphenols (56.8 mg) in mildly hypercholesterolemic subjects for 45 days | ↑Roseburia spp., ↑Prevotella spp., ↑Paraprevotella spp., ↑Dialister succinatiphilus, ↑Roseburia hominis, ↑Butyrivibrio crossotus, ↑B. pseudocatenulatum,* ↑Clostridium symbiosum, ↓Megamonas hypermegale, ↓Clostridium nexile, ↓Roseburia inulinivorans | ↑A. muciniphila, correlated with ↓HDL-C | ↓Dyslipidemia, ↑propionate, and ↑acetate | [171] |
| Flavan-3-ols and stilbenes | Epigallocatechin-3-gallate (EGCG) + resveratrol (RSV) | Intake of EGCG (282 mg/day) and RES (80 mg/day) in overweight and obese men and women for 12 weeks | ↓Bacteroidota in men | No changes were detected in A. muciniphila | Improved fat oxidation in men | [172] |
| Resveratrol (RSV) | Intake of 1g of RSV twice daily in obese men with MetS for 30 days | ↓Alistipes, ↓Collinsiella, ↓Christensenella, ↓Holdemania, and ↓Turicibacter spp. in Caucasian men | ↑A. muciniphila only in Caucasian men | Improved glucose homeostasis only in Caucasian men | [161] | |
| Magnolia berry Schisandra Chinensis (SCF) | Intake of 100 mL of juice twice a day containing 12 mg total phenolics and 3.34 mg total flavonoids in obese woman for 12 weeks | ↑Roseburia, ↑Prevotella, ↑Bifidobacterium,* and ↑Bacteroides* spp. | ↑A. muciniphila | ↓Fat mass, ↓fasting blood glucose, ↓TG, ↓AST, and ↓ALT | [173] | |
| Flavanones | Orange juice (hesperidin) | Intake of orange juice (300 mL d−1) for 60 days in healthy women | ↑Lactobacillus spp.,* ↑Ruminococcus spp. | ↑A. muciniphila, correlated with improved glycemia and dyslipidemia | ↓Glucose intolerance, ↓insulin resistance, ↓TG | [40] |
| Flavan-3-ols | Mango (quercetin and kaempferol glycosides, gallic acid and gallotannins) | Intake of 300–400 g of mango pulp in patients with IBD for 8 weeks | ↑ L. plantarum,* ↑L. lactis, ↑ L. reuteri* | No changes were detected in A. muciniphila | ↓Plasma levels of proinflammatory cytokines, ↑butyrate | [174] |
| Pomegranate juice (PJ) (Ellagitannins) and urolithin A (UA) | Intake of PJ (240 mL) for 3 weeks and UA (500 mg) for 48 h in healthy subjects | ↓F/B and ↑microbial diversity, ↑Clostridiales, and ↑Ruminococcaceae in UA producers; ↑F/B and ↓in non-UA producers | ↑A. muciniphilia in high UA producers | 6.0-fold ↑plasma UA glucuronide after UA intake compared to PJ | [42] | |
| Pomegranate extract | Intake of POM (1 g/day) in healthy volunteers for 4 weeks categorized as urolithin A (UA) producers and non-producers | ↑Actinobacteria, ↓Firmicutes, ↑Butyrivibrio spp., ↑Enterobacter spp., ↑Escherichia spp., ↑Lactobacillus spp.,* ↑Prevotella spp., ↓Collinsella spp. in UA producers | 47-fold ↑A. muciniphilia in UA producers | ↑Plasma UA glucuronide | [43,52] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Daza, M.C.; de Vos, W.M. Polyphenols as Drivers of a Homeostatic Gut Microecology and Immuno-Metabolic Traits of Akkermansia muciniphila: From Mouse to Man. Int. J. Mol. Sci. 2023, 24, 45. https://doi.org/10.3390/ijms24010045
Rodríguez-Daza MC, de Vos WM. Polyphenols as Drivers of a Homeostatic Gut Microecology and Immuno-Metabolic Traits of Akkermansia muciniphila: From Mouse to Man. International Journal of Molecular Sciences. 2023; 24(1):45. https://doi.org/10.3390/ijms24010045
Chicago/Turabian StyleRodríguez-Daza, María Carolina, and Willem M. de Vos. 2023. "Polyphenols as Drivers of a Homeostatic Gut Microecology and Immuno-Metabolic Traits of Akkermansia muciniphila: From Mouse to Man" International Journal of Molecular Sciences 24, no. 1: 45. https://doi.org/10.3390/ijms24010045
APA StyleRodríguez-Daza, M. C., & de Vos, W. M. (2023). Polyphenols as Drivers of a Homeostatic Gut Microecology and Immuno-Metabolic Traits of Akkermansia muciniphila: From Mouse to Man. International Journal of Molecular Sciences, 24(1), 45. https://doi.org/10.3390/ijms24010045