1. Introduction
Quinazolin-4-ones and -2,4-diones (Quizones) have been well documented as an important template of potential pharmacological and biochemical interests. Relevant studies of
Ketanserin in these areas have strongly suggested that these derivatives can be used for treating hypertensive symptoms by reducing peripheral vascular resistance by blocking the effect of serotonin functions (
Figure 1) [
1,
2]. In addition, an amide variant TCMDC-125133 bearing a chiral α-Quizone group was found to exhibit effective antimalarial activity against the
Plasmodium falciparum 3D7 strain (
Figure 1) [
3,
4].
In 2012, J.-W. Chern et al. introduced 3-hydroxy/benzyloxy and 2-carbamoyl groups into the bicyclic skeleton of 1,3-quinazolin-4-ones (Quiz) as potential pharmacophores [
5]. These derivatives can be used as candidates for HCV NS5B polymerase inhibition. In particular, they serve to block the synthesis of hepatitis C virus RNA, thus inhibiting the replication of the virus (
Figure 2). In 2016, P. Verhaeghe, P. Vanelle, et al. introduced 4-benzyloxy/4-amino and 2-trichloromethyl groups into the 1,3-quinazolines [
6,
7]. The resulting compounds as well as 2-amino analogs [
8] may serve as inhibitors for histone (lysine) methyltransferase (HMT). They may thus inhibit the mechanism of G9a during metastasis of cancer cells in vivo. Among 35 different analogs tested, those containing (chiral) 4-benzyl-oxy and -amino groups showed interesting anti-Plasmodium activities [
6].
Quinazoline derivatives were found to exhibit good inhibitory activities against the epidermal growth factor receptor (EGFR) by X. Wu, J. Zhang, et al. [
9] Notably, certain lung cancer cells tend to evolve with EGFR mutations, which cause their abnormal proliferation and thus accelerate cancer progression. By modifying the 4-arylamino groups to (chiral) 4-benzyl-oxy and -amino groups, it was found that they possessed similar inhibition activities towards EGFR-TKs. Therefore, they may also serve as potential anti-tumor drug candidates.
On the other hand, 4-oxo-3-aryl-2-methyl/benzylthio-Quiz have shown remarkable dihydrofolate reductase (DHFR) and EGFR-TK inhibitions at 0.1–0.9/0.2–1.8 μM (SMe/SBn) and ---/13.4 nM (SMe/SBn) ranges, respectively, indicating the unique role of the 2-mercapto groups [
10]. Furthermore, acetohydroxyacid synthase (AHAS) constitutes the first key catalytic step of biosynthesis toward branched-chain amino acids and acts as a potential drug candidate against
Mycobacterium tuberculosis (MTB). 4-Oxo-2-aryl-3-benzoyloxy-Quiz were identified as promising inhibitors of MTB-AHAS [
11]. Serine protease DegS play a key role in the outer membrane stress response pathway for bacteria. It was believed that covalently modifying lysine residues to Serine protease DegS allows for its unique allosteric activation. Similarly, 3-benzoyloxy-quinazoline analogs also may function as allosteric activators for Degs by lysine benzoylation [
12].
Very recently, efficient racemic synthesis of chiral 3-benzyloxy-1,3-quinazoline-4-ones were reported by using Cu(II)/
t-butyl hydroperoxide combination to catalyze oxidative anchoring of quinazoline-3-oxides to the benzylic positions of various aryl containing compounds (
Scheme 1) [
13].
In view of the documented 2-H, 2-NH
2, and 2-carboxyl equivalent (i.e., amide and CCl
3) groups in the 3-hydroxy-quinazolinone type drug candidates and potential reversible benzyl group shift [from N-O to C(O)], we sought to examine a series of 3OH-Quiz systems bearing 2-H and 2-SMe-groups as a new type of radical trapping agent (
Figure 3) based on our previous vanadyl complex-catalyzed 1,2-oxytrifluoromethylation protocol with
N-hydroxyphthalimide (NHPI) [
14,
15,
16]. The compatibility of the imine N1, 2-hydroxy, and thio units towards incipient radical species during the reaction will be determined. In addition, 2-phenyl-3hydroxy-Quiz was included as a new type of 2-group in drug candidate design. We report herein the preliminary successful results of asymmetric 1,2-oxy-trifluoromethylation.
2. Results
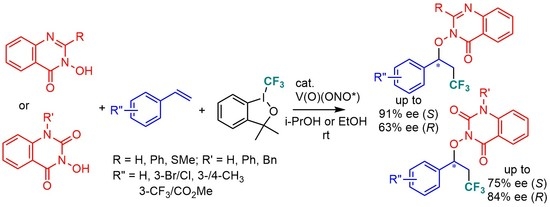
Based on our previous experience, we selected the two best vanadyl complexes, V(O)-1 and V(O)-2 bearing 3-(2,5-dimethyl)phenyl-5-bromophenyl (i.e., 3-DMP-5-Br) and 3-(tert-butyl)-5-bromophenyl (i.e., 3-tert-Bu-5-Br) groups, respectively, as the N-salicylidene-tert-leucinate templates for the current study in order to attain complementary results in terms of enantiocontrol. 3-Bromostyrene was chosen as the best standard substrate for the asymmetric cross-coupling reactions with these two catalysts in i-propanol at ambient temperature. In addition, both 3-methyl and 4-methylstyrenes were examined for comparison.
When the 3-DMP-5-Br vanadyl complex
V(O)-1 was used as the catalyst, the cross-coupling reactions proceeded smoothly in 68 h and 84 h, respectively, with 2-H and 2-Ph-substituted 3OH-Quizs
1 and
2 (entries 1 and 3,
Table 1). The corresponding products
8a and
9a, enriched in
R-enantiomers, were isolated in 71 and 45% yields and in 63% and 50% ees, respectively. In the case of 2-SMe substituted 3OH-Quiz
3 (entry 4), the corresponding product, (
R)-
9a, was isolated with a 62% yield and in 38% ee in 30 h. Notably, the use of 2-thioxo substituted 3OH-Quiz
7 as the radical trapping agent led to its dramatic decomposition, presumably due to interrupting thio trapping (entry 13). The corresponding desired product
14a was isolated in only 4% yield and in 60% ee (
R). In both series, the reaction efficiency, yields, and extent of asymmetric induction decreased with increasing steric sof the 2-substituents (i.e., H was better than Ph and C=S was better than SMe).
In the cases of 2-Ph-3OH-Quiz 2 as the trapping agent with 3-methyl and 4-methyl styrenes as the substrates (entry 2), the reactions proceeded with shorter reaction times of 70 and 52 h (cf 84 h in entry 3), respectively. The corresponding products 9b and 9c were obtained at higher yields of 60% and 70% (cf 45% yield in entry 3), but with lower asymmetric inductions of 45% and 26% ees (cf 50% ee in entry 3), respectively.
For a much stricter comparison, 3-hydroxy-1-H- and 1-phenylquinazoline-2,4(1H,3H)-diones 4 and 5 (i.e., 1-H- and 1-Ph-3OH-Quizdiones) were utilized. For 3-Br- and 3-Cl-styrenes (entry 7) with 4, the reactions went to completion in much shorter reaction time of 43 h/18 h (cf 68 h in entry 1). The corresponding products 11a and 11a’ furnished much higher yields of 88/83% (cf 71% yield in entry 1), respectively, and improved asymmetric induction of 77/72% ee (cf 63% ee in entry 1), respectively. For the 3-Br-styrene (entry 9) with 5, the reaction went to completion in a much shorter reaction time of 18 h (cf 84 h in entry 3). The corresponding product 12a was furnished at a higher yield of 93% (cf 45% yield in entry 3) and improved asymmetric induction of 68% ee (cf 50% ee in entry 3).
A trend similar to that of 4 was observed for styrene (4-H) and 3-methyl-styrene cases (entries 5 and 6). Both of the reactions went to completion in much shorter reaction times of 51 and 38 h (cf 68 h in entry 1), respectively. The corresponding products 11b and 11c were isolated at higher yields of 84 and 83% (cf 71% yield in entry 1), respectively, and with comparable to improved asymmetric inductions of 63 and 71% ees (cf 63% ee in entry 1), respectively.
Cross-couplings of 5 with 3-methyl and 4-methyl-styrene cases (entry 8), both the reactions also went to completion in much shorter reaction time of 17 h and 24 h (cf 70 h and 52 h in entry 2), respectively. The corresponding products 12b and 12c were isolated at comparable or higher yields of 59/80% (cf 60/70% yields in entry 2), respectively, and with improved asymmetric inductions of 77/48% ee (cf 45/26% ee in entry 3), respectively. In the 3-methylstyrene case, an even better yield (83%) and asymmetric induction (82% ee) can be achieved by carrying out the reaction in EtOH.
Since the uses of 1-H- and 1-Ph-3OH-Quizdiones 4 and 5 as the radical trapping agents led to similar results (cf entries 6–7 and 8–9), 1-benzyl-Quizdione 6 was further synthesized and examined. In the case of 3-bromostyrene (first set of data in entry 10), the corresponding product 13a was isolated in 91% which was comparable to 93% yield in 1-Ph case (entry 9), albeit with much longer reaction time of 44 h (cf 18 h in entry 9). However, a much better enantioselectivity of 84% ee (R) was observed (cf 68% ee in entry 9). Comparable asymmetric induction of 81% ee (R) was also achieved with 3-chlorostyrene (second set of data in entry 10). The desired product 13a′ was obtained with an 83% yield in 65 h. Further studies with 3-trifluoromethyl and 3-carbomethoxy-styrenes led to the cross-coupling products 13b and 13b′ with similarly satisfactory yields (88 and 90%, respectively) and asymmetric induction (82 and 81% ees in entries 11 and 12, respectively).
Therefore among seven different Quizs and Quizdiones examined, 1-benzyl-Quizdione 6 showed the best results (83–91% yields and 81–84% ees) by 3-DMP-5-Br catalyst V(O)-1.
When the 3-
tert-Bu-5-Br vanadyl complex
V(O)-2 was used as the catalyst (entries 14–23,
Table 1), the cross-coupling reactions proceeded somewhat sluggishly in 4–5 days with 2-H and 2-Ph-substituted 3OH-Quizs
1 and
2 (entries 14 and 16). The corresponding products
7a and
8a, enriched in complementary
S-enantiomers were isolated in 31 and 28% yields, respectively, but in comparable or much higher asymmetric induction of 66% and
91% ees, respectively (cf 63% and 50% ees in entries 1 and 3). In the case of 2-SMe substituted 3OH-Quiz case
3 (entry 17), the corresponding product (
S)-
9a was isolated at a lower yield of 39% (cf 62% yield in entry 4) and a comparable asymmetric induction of 50% ee (cf 38% ee in entry 4) in 50 h. Again, the use of 2-thioxo substituted 3OH-Quiz
7 as the radical trapping agent led to its dramatic decomposition presumably due to interrupting thio trapping. No desired product was isolated. In marked contrast, an opposite trend was observed in the former series (i.e., 2-Ph better than 2-H). Therefore, one would expect a unique and advantageous π-π interaction in the former series when catalyzed by 3-
tert-5-By vanadyl complex
V(O)-2 (vide infra).
In the cases of 2-Ph-3OH-Quiz 2 as the trapping agent with 3-methyl and 4-methyl styrenes as the substrates (entry 15), the reactions also proceeded with a longer reaction time of 4–4.5 days (cf 70/52 h in entry 2). The corresponding products 8b and 8c were obtained at lower yields of 31% and 55% (cf 60/70% yields in entry 2), respectively, but with much improved asymmetric inductions of 88 and 85% ees (cf 45/26% ees in entry 2), respectively.
For a fair comparison, 1-H- and 1-Ph-3OH-Quizdiones 4 and 5 were similarly examined. For the 3-Br and 3-Me-styrenes with 4 (entries 18–20), the reaction went to completion in a prolonged reaction time of 5 days (cf 38 and 48 h in entries 6–7, 30 h in entry 4, and 68 h in entry 1). The corresponding products 11a-c were provided at satisfactory yields of 53–72% (cf 83–88% yields in entries 5–7, 62% yield in entry 4, and 28% yield in entry 16) and comparable to higher asymmetric inductions of 69–75% ees (cf 63–77% ee in entries 5–7, 63% ee in entry 1, and 38% ee in entry 4).
For 3-Me- and 4-Me-styrenes with 5 (entry 21), the reaction went to completion in a prolonged reaction time of 5.4/4 days (cf 17/24 h in entry 8 and 108 h in entry 15), respectively. The corresponding products 12b/21c were provided in satisfactory yields of 56/64% (cf 31/55% yields in entry 15) and a much lower asymmetric induction of 64/74% ees (cf 88/85% ees in entry 15). A better asymmetric induction of 75% ee can be similarly achieved by performing the reaction in EtOH (cf entries 21 and 8). For 3-Br-styrene with 5 (entry 22), the reaction went to completion in 96 h even in EtOH (cf 18 h in entry 9 and 92 h in entry 16). The corresponding product 11a was obtained with a better yield of 46% (cf 28% yield in entry 16) but with a lower asymmetric induction of 72% ee (cf 91% ee in entry 16). Evidently, the position and orientation of the phenyl substituent (cf 2-Ph and 1-Ph, entries 16 and 21) play a decisive role in asymmetric induction during the radical trapping events (see below). In comparison, the catalyst V(O)-2 was used to investigate 1-benzyl-Quizdione 6 with 3-bromostyrene. The reaction went to completion in 5 days, and the expected product 13a was isolated in 45% yield and 74% ee (entry 23).
Therefore, among the three Quizzes 1–3 examined, Ph-Quiz 2 led to the best asymmetric induction of 85–91% ees. On the other hand, all of three Quizdiones 4–6 led to similar levels of asymmetric induction of up to 74–75% ees by the V(O)-2 catalyst.
3. Discussions
Based on our previous study using NHPI as the radical trapping agent,
9 one would expect that 3OH-Quiz (
1–
3) and 3OH-Quizdione (
4–
6) first undergo facile exchanges with the methoxide and methanol ligands. In the 3OH-Quiz case for both studied vanadyl complexes, they would form the corresponding vanadyl-bound bidentates, 3OH-Quiz-
15 and 3OH-Quiz-
16, respectively (
Figure 4). The relative energies for 3-DMP and 3-
tert-Bu complexes chelated by
1 by energy minimization were 37.7 and 40.1 kcal/mol, respectively. The higher energy (and thus less stability) in the latter complex-
16 may be attributed to the unfavorable steric interaction between the
tert-butyl group and the 3OH-Quiz chelates (
1–
3) in view of the C-
H to
N-O vDW repulsion. This weakness explains why the reaction time was much longer and in a range of 50 h to 4–5 days (cf 30 h to 2–3.5 days by using 3-DMP catalyst). A similar trend was observed in the corresponding 3OH-Quizdione (
4–
6) chelates, the reaction time was much longer ranging from 5–5.4 days (cf 17/24 for 1-Ph and 38/51 h for 1-H by using 3-DMP catalyst).
A series of theoretical calculations were carried out by placing the incipient 3-Br-styrene-derived benzylic radical first directly in front of the bound 3OH-Quiz chelate (
I) followed by a slight shift to the left and proximal to the 3-DMP group with the CF
3CH
2 group away from the axial vanadyl (i.e., V=O) unit (
II),
Figure 5. A weak interaction between the benzylic radical and the 3-
O(N) unit was then introduced. In view of the favorable π-π -π interaction, further slide in between 3-DMP and the bound 3OH-Quiz chelate in a sandwich manner to form
III is preferred.
Based on the progressive snap shots obtained by tracing the approaching benzylic radical to the bound 3OH-Quiz chelate, it was found that the 2-phenyl group would encounter much more severe steric repulsion during the sliding progress of the benzylic radical into a sandwich stacking. Therefore, the overall energy of the 2-phenyl system
Rad---V(O)-2 (left shift) was 14.6 kcal/mol higher than that of the 2-H system
Rad---V(O)-1 (left shift) (i.e., 53.43 kcal/mol vs 38.83 kcal/mol),
Figure 6A. As a result, the approaching radical prefers a slight shift to the right to avoid the steric repulsion of the bound 3OH-Quiz chelate [i.e.,
Rad---V(O)-1 (right shift)]. Under such circumstances, the overall energy of the 2-phenyl system
Rad---V(O)-2 (right shift) dramatically decreased to 44.39 kcal/mol (
Figure 6B) in view of the attractive π-π interaction. Therefore, the enantioselectivity in the 2-phenyl system dropped to 50% ee (
R) since a competing approach of the incipient radical directly from the right-hand side proximal to the vanadyl carboxyl side would lead to the opposite (
S)-enantiomer.
The best enantiocontrol in the 1-H-2-C(O) system (77% ee
R in entry 7,
Table 1) can be rationalized in terms of its electron-withdrawing nature and its hydrogen-bonding type non-covalent interaction (NCI) with the methylene H of the CH
2CF
3 group. The overall energy of the 1-H-2-C(O) system
Rad---V(O)-3 (left shift) (21.43 kcal/mol) was the lowest (
Figure 7A).
In contrast, the worst enantiocontrol in the 2-SMe system can be explained in terms of the unfavorable steric repulsion between the approaching benzylic radical and the methylthio group, particularly in conformation-2 (33.52 kcal/mol) in
Rad---V(O)-4 (left shift),
Figure 7A. As a result, the approaching radical would prefer to slightly shift to the right to avoid the steric repulsion to the bound 3OH-Quiz chelate [i.e.,
Rad---V(O)-4 (right shift)]. Under such circumstances, the overall energy of the 2-SMe system significantly decreased to 27.64 kcal/mol (
Figure 7B) in view of the attractive π-π interaction. Therefore, the enantioselectivity in the 2-SMe system dropped to only 38% ee (
R) since a similar approach from the right leading to the opposite (
S)-enantiomer would also compete.
The significant drop in enantioselectivities in the 2-phenyl systems caused by switching the substrate to 3-methyl and 4-methylstyrenes can be envisioned in terms of the increasing steric repulsions between the 3-Me/4-Me groups and the 5-methyl group in DMP during the initial front approach en route to their left or right shift (
Figure 8).
The higher enantioselectivity of
11a (cf 68% ee
R in entry 9 and 50% ee in entry 3) observed in the 1-Ph-2-oxo system
Rad---V(O)-5 (left shift) can also be rationalized in view of the favorable H-bonding type NCI and absence of steric repulsion between the 1-phenyl group and the 3-Br-phenyl group in the benzyl radical (
Figure 9A). Alternatively,
Rad---V(O)-5 (right shift) can also be operative with similar enantio-control in view of its similar overall energy (
Figure 9C). The even better asymmetric induction in
13a (84% ee in entry 10) may be visualized by the
Rad---V(O)-6 (left shift) interaction model (
Figure 9B) but with an additional
N-benzyl CH for π interaction. Both the absolute stereochemistries of the corresponding products
12a and
13a were confirmed to be (
R) by X-ray crystallographic analyses of their recrystallized products in almost enantiomerically pure forms (
Figure 9D). The selected crystal data and structure refinement for (
R)-
12a and
13a are included as
Table S1 and the first paragraph on page S34. Their Platon drawings are included as
Figures S1 and S2).
In the 3-
tert-butyl-5-Br vanadyl catalyst
V(O)-2 system, the best result observed in the 2-phenyl case (91% ee in entry 13) can be rationalized in terms of the favorable π-π interaction between the 2-phenyl group and the 3-Br-phenyl ring in the benzylic radical as shown in the optimized geometries of the catalyst (
Figure 10A) and the interaction of the vanadyl-bound 2-Ph-Quiz with the benzylic radical (
Figure 10B). The significant drop in enantio-selectivity in the 1-phenyl-2C(O) case (56% ee) can thus be understood to be due to the lack of π-π interaction. The lower asymmetric induction in 2-SMe (50% ee in entry14) also supports the decisive π-π interaction in the 2-phenyl case. In addition, a slightly better asymmetric induction in 1-Ph-2-C(O) system with catalyst
V(O)-1 (cf 68% ee and 56% ee in entries 10 and 18) supports a slightly better π-π interaction in
Rad---V(O)1-5 as compared to
Rad---V(O)2-5 (cf
Figure 9A and
Figure 10B). A similar range of asymmetric inductions in 1-H-2-C(O) system for both
V(O)-1 and
V(O)-2 catalysts (cf 63–77% ees and 69–75% ees) also supports a participating role for π-π interaction in the 1-Ph-2-C(O) system (i.e., entries 10 and 18).
5. Conclusions
We have documented a new type of asymmetric 1,2-oxytrifluoromethylation of styrenes catalyzed by our two vanadyl complexes V(O)-1 (3-DMP) and V(O)-2 (3-t-Bu), in a complementary manner with various 1- and 2-substituted-3-hydroxy-1,3-quinazolin(ones) [(3(OH)Quiz(one)] as radical trapping agents. The imine and carbamate units in these substrates were confirmed to be compatible with CF3 radicals and benzylic radicals. Among three different 2-substituted-(3(OH)Quizs (i.e., H, Ph, and SMe) examined in i-propanol except the 2-phenyl case by the catalyst V(O)-2, the extents of asymmetric induction decreased with increasing sterics of the 2-substituents [i.e., 2-H (63/66% ee) > 2-Ph (45/50% ees) > 2-SMe (38/50% ees)]. The corresponding (R)- and (S)-cross-coupling products were obtained in up to 71% and 55% yields for p-/m-methylstyrenes and m-bromostyrenes in up to (R)-63% ee (in 2-H case) and (S)-91% ee (in 2-Ph case by catalyst V(O)-2), respectively. Among three different 1-substituted-3(OH)Quiz-2-ones (i.e., H, Ph, and Bn) examined, 1- Bn-Quiz-2-one 6 showed the best results (83–91% yields and 81–84% ees) by the 3-DMP-5-Br catalyst V(O)-1. In marked contrast, the results from catalysts V(O)-1 and V(O)-2 by using 3-hydroxy-1-H and 3-hydroxy-1-phenylquinazoline-2,4-diones as the trapping agents were found to be less enantioselective. The best results in the m-bromo-products were obtained in 88/93% and 53/46% yields and in 77/68% ees (R) and 74/72% ees (S), respectively, despite with some improvement in EtOH.
Theoretical calculations were performed to rationalize the experimental trends. In the former case, the incipient benzylic radical after CF
3 radical addition to a given styrene substrate approaches the vanadyl-bound [(3OH)Quiz(one)] from the side proximal to the 3-DMP group in the catalyst in a preferred sandwich type DMP (C-H)-π-π interaction. In contrast, the benzylic radical approaches [V(O)-(3OH)Quiz(one)] from the side opposite to the sterically demanding 3-
tert-butyl group, thus leading to a complementary enantiocontrol. In the 2-phenyl-(3OH)Quiz case, the steric interaction of the two interrupting π-π systems between the incipient 3-Br-benzylic radical and the vanadyl-bound 2-phenyl-[V(O)-(3OH)Quiz] was identified to be responsible for the decreased enantioselectivities to (
R)-50% ee by using a 3-DMP5-Br based catalyst. In contrast, the best enantioselectivities of (
S)-85–91% ees were achieved using the
V(O)-2 catalyst in terms of favorable π-π interactions with its approach from the right hand side. Conversely in the 1-H-2-C(O) system, the asymmetric inductions were similar in up to 75–77% ees with both catalysts in view of the lack of 2-Ph or 1-Ph group. Therefore, the 2-Ph- and 1-Ph/Bn -2-C(O)-(3OH)Quiz(one)s serve as the best radical trapping agents, which bode well for their potential applications as key intermediates for acetohydroxyacid synthase and EGFR kinase inhibitors. Either enantiomeric products and racemic mixtures will be subjected to the sequences of enzymatic and/or biomedicinal tests to gain insight into the effect of absolute chirality to biomedical activities (see
Table S2 for their preliminary single point analyses).