Human Hemoglobin and Antipsychotics Clozapine, Ziprasidone and Sertindole: Friends or Foes?
Abstract
:1. Introduction
2. Results and Discussion
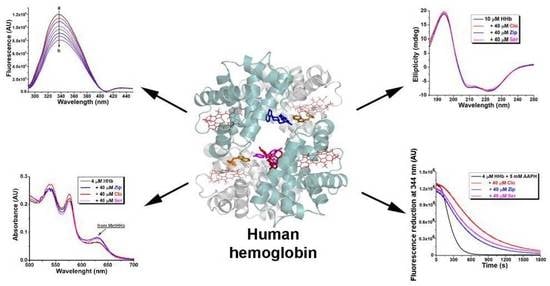
2.1. Fluorescence Quenching of Human Hemoglobin by Atypical Antipsychotics
2.2. Binding Constants Determination in Human Hemoglobin and Antipsychotic Systems
2.3. Synchronous Fluorescence Measurements in Human Hemoglobin and Antipsychotic Systems
2.4. Thermodynamic Study of Antipsychotics Binding to Human Hemoglobin
2.5. Docking Results
2.6. Circular Dichroism Spectra Analysis
2.7. Effect of Atypical Antipsychotics on the Thermal Stability of Human Hemoglobin
2.8. Effect of Atypical Antipsychotics on Spontaneous Human Hemoglobin Oxidation
2.9. Effect of Atypical Antipsychotics on Oxidant-Assisted Human Hemoglobin Oxidation
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Fluorescence Measurements
3.2.2. Circular Dichroism (CD) Measurements
3.2.3. Visible Absorbance (Vis) Measurements
3.2.4. Statistical Analysis
3.2.5. Molecular Docking
3.3. Theory and Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsia, C.C. Respiratory function of hemoglobin. N. Engl. J. Med. 1998, 338, 239–247. [Google Scholar] [CrossRef]
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Remington, G.; Lee, J.; Agid, O.; Takeuchi, H.; Foussias, G.; Hahn, M.; Fervaha, G.; Burton, L.; Powell, V. Clozapine’s critical role in treatment resistant schizophrenia: Ensuring both safety and use. Expert Opin. Drug Saf. 2016, 15, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Komossa, K.; Rummel-Kluge, C.; Hunger, H.; Schwarz, S.; Bhoopathi, P.S.S.; Kissling, W.; Leucht, S. Ziprasidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst. Rev. 2009, 4, CD006627. [Google Scholar] [CrossRef] [PubMed]
- Siskind, D.; Gallagher, E.; Winckel, K.; Hollingworth, S.; Kisely, S.; Firth, J.; Correll, C.U.; Marteene, W. Does Switching Antipsychotics Ameliorate Weight Gain in Patients With Severe Mental Illness? A Systematic Review and Meta-analysis. Schizophr. Bull. 2021, 47, 948–958. [Google Scholar] [CrossRef]
- Zoccali, R.A.; Bruno, A.; Muscatello, M.R.A. Efficacy and safety of sertindole in schizophrenia: A clinical review. J. Clin. Psychopharmacol. 2015, 35, 286–295. [Google Scholar] [CrossRef]
- Wallace, S.M.; Reigelman, S. Uptake of acetazolamide by human erythrocytes in vitro. J. Pharm. Sci. 1977, 66, 729–731. [Google Scholar] [CrossRef]
- Villa, C.H.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016, 106, 88–103. [Google Scholar] [CrossRef]
- Perutz, M.F.; Fermi, G.; Abraham, D.J.; Poyart, C.; Bursaux, E. Hemoglobin as a receptor of drugs and peptides: X-ray studies of the stereochemistry of binding. J. Am. Chem. Soc. 1986, 108, 1064–1078. [Google Scholar] [CrossRef]
- Nedić, O.; Penezić, A.; Minić, S.; Radomirović, M.; Nikolić, M.; Ćirković Veličković, T.; Gligorijević, N. Food Antioxidants and Their Interaction with Human Proteins. Antioxidants 2023, 12, 815. [Google Scholar] [CrossRef]
- Alpert, B.; Jameson, D.M.; Weber, G. Tryptophan emission from human haemoglobin and its isolated subunits. J. Photochem. Photobiol. 1980, 31, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mueser, T.C.; Rogers, P.H.; Arnone, A. Interface sliding as illustrated by the multiple quaternary structures of liganded hemoglobin. Biochemistry 2000, 39, 15353–15364. [Google Scholar] [CrossRef]
- Hirsch, R.E.; Nagel, R.L. Conformational studies of hemoglobins using intrinsic fluorescence measurements. J. Biol. Chem. 1981, 256, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Dohare, N.; Siddiquee, M.A.; Parray, M.D.; Kumar, A.; Patel, R. Esterase activity and interaction of human hemoglobin with diclofenac sodium: A spectroscopic and molecular docking study. J. Mol. Recognit. 2020, 33, e2841. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Ware, W.R. Oxygen quenching of fluorescence in solution: An experimental study of the diffusion process. J. Phys. Chem. 1962, 66, 455–458. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, H.; Bao, W.; Zou, G. Studies on the interaction between docetaxel and human hemoglobin by spectroscopic analysis and molecular docking. J. Photochem. Photobiol. B 2011, 105, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Tunç, S.; Cetinkaya, A.; Duman, O. Spectroscopic investigations of the interactions of tramadol hydrochloride and 5-azacytidine drugs with human serum albumin and human hemoglobin proteins. J. Photochem. Photobiol. B 2013, 120, 59–65. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O.; Bozoğlan, B.K. Studies on the interactions of chloroquine diphosphate and phenelzine sulfate drugs with human serum albumin and human hemoglobin proteins by spectroscopic techniques. J. Lumines 2013, 140, 87–94. [Google Scholar] [CrossRef]
- Maurya, N.; Ud Din Parray, M.; Maurya, J.K.; Kumar, A.; Patel, R. Interaction of promethazine and adiphenine to human hemoglobin: A comparative spectroscopic and computational analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 199, 32–42. [Google Scholar] [CrossRef]
- Dufour, C.; Dangles, O. Flavonoid-serum albumin complexation: Determination of binding constants and binding sites by fluorescence spectroscopy. Biochim. Biophys. Acta 2005, 1721, 164–173. [Google Scholar] [CrossRef]
- Undeland, I.; Kristinsson, H.G.; Hultin, H.O. Hemoglobin-mediated oxidation of washed minced cod muscle phospholipids: Effect of pH and hemoglobin source. J. Agric. Food Chem. 2004, 52, 4444–4451. [Google Scholar] [CrossRef] [PubMed]
- Seal, P.; Sikdar, J.; Roy, A.; Haldar, R. Acetaminophen interacts with human hemoglobin: Optical, physical and molecular modeling studies. J. Biomol. Struct. Dyn. 2017, 35, 1307–1321. [Google Scholar] [CrossRef]
- Miller, J.N. Recent advances in molecular luminescence analysis. Proc. Anal. Div. Chem. Soc. 1979, 16, 203–208. [Google Scholar]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Nishi, K.; Yamasaki, K.; Otagiri, M. Serum Albumin, Lipid and Drug Binding. Subcell. Biochem. 2020, 94, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Wang, Y.-Q.; Jiang, M.-L. A fluorimetric study of the interaction of C.I. Solvent Red 24 with haemoglobin. Dye. Pigm. 2009, 82, 156–163. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar] [CrossRef]
- Stadler, A.M.; Garvey, C.J.; Bocahut, A.; Sacquin-Mora, S.; Digel, I.; Schneider, G.J.; Natali, F.; Artmann, G.M.; Zaccai, G. Thermal fluctuations of haemoglobin from different species: Adaptation to temperature via conformational dynamics. J. R. Soc. Interface 2012, 9, 2845–2855. [Google Scholar] [CrossRef]
- Levy, A.; Kuppusamy, P.; Rifkind, J.M. Multiple heme pocket subconformations of methemoglobin associated with distal histidine interactions. Biochemistry 1990, 29, 9311–9316. [Google Scholar] [CrossRef]
- Szebeni, J.; Winterbourn, C.C.; Carrell, R.W. Oxidative interactions between haemoglobin and membrane lipid. A liposome model. Biochem. J. 1984, 220, 685–692. [Google Scholar] [CrossRef]
- Dalla Libera, A.; Scutari, G.; Boscolo, R.; Rigobello, M.P.; Bindoli, A. Antioxidant properties of clozapine and related neuroleptics. Free Radic. Res. 1998, 29, 151–157. [Google Scholar] [CrossRef]
- Caruso, G.; Grasso, M.; Fidilio, A.; Tascedda, F.; Drago, F.; Caraci, F. Antioxidant Properties of Second-Generation Antipsychotics: Focus on Microglia. Pharmaceuticals 2020, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Kosmachevskaya, O.V.; Topunov, A.F. Alternate and additional functions of erythrocyte hemoglobin. Biochemistry 2018, 83, 1575–1593. [Google Scholar] [CrossRef] [PubMed]
- Vogel, F.; Gansmüller, R.; Leiblein, T.; Dietmaier, O.; Wassmuth, H.; Gründer, G.; Hiemke, C. The use of ziprasidone in clinical practice: Analysis of pharmacokinetic and pharmacodynamic aspects from data of a drug monitoring survey. Eur. Psychiatry 2009, 24, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F.; de Sena, E.P.; de Oliveira, I.R. Sertindole in the Management of Schizophrenia. J. Cent. Nerv. Syst. Dis. 2011, 3, 75–85. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Betigeri, S.; Thakur, A.; Raghavan, K. Use of 2,2′-azobis(2-amidinopropane) dihydrochloride as a reagent tool for evaluation of oxidative stability of drugs. Pharm. Res. 2005, 22, 310–317. [Google Scholar] [CrossRef]
- Brinholi, F.F.; de Farias, C.C.; Bonifácio, K.L.; Higachi, L.; Casagrande, R.; Moreira, E.G.; Barbosa, D.S. Clozapine and olanzapine are better antioxidants than haloperidol, quetiapine, risperidone and ziprasidone in in vitro models. Biomed. Pharmacother. 2016, 81, 411–415. [Google Scholar] [CrossRef]
- Gligorijević, N.; Vasović, T.; Lević, S.; Miljević, Č.; Nedić, O.; Nikolić, M. Atypical antipsychotic clozapine binds fibrinogen and affects fibrin formation. Int. J. Biol. Macromol. 2020, 154, 142–149. [Google Scholar] [CrossRef]
- Šunderić, M.; Vasović, T.; Milčić, M.; Miljević, Č.; Nedić, O.; Nikolić, M.R.; Gligorijević, N. Antipsychotic clozapine binding to alpha-2-macroglobulin protects interacting partners against oxidation and preserves the anti-proteinase activity of the protein. Int. J. Biol. Macromol. 2021, 183, 502–512. [Google Scholar] [CrossRef]
- Chang, W.H.; Lin, S.K.; Lane, H.Y.; Hu, W.H.; Jann, M.W.; Lin, H.N. Clozapine dosages and plasma drug concentrations. J. Formos. Med. Assoc. 1997, 96, 599–605. [Google Scholar]
- Miller, D.D. Review and management of clozapine side effects. J. Clin. Psychiatry 2000, 61 (Suppl. S8), 14–17; discussion 18–19. [Google Scholar]
- Tentori, L.; Salvati, A.M. Hemoglobinometry in human blood. In Methods in Enzymology; Antonini, E., Rossi-Bernardi, L., Hiancone, E., Eds.; Academic Press: New York, NY, USA, 1981; pp. 707–715. [Google Scholar] [CrossRef]
- Gligorijević, N.; Minić, S.; Radibratović, M.; Papadimitriou, V.; Nedić, O.; Sotiroudis, T.G.; Nikolić, M.R. Nutraceutical phycocyanobilin binding to catalase protects the pigment from oxidation without affecting catalytic activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 251, 119483. [Google Scholar] [CrossRef]
- Minic, S.; Stanic-Vucinic, D.; Radomirovic, M.; Radibratovic, M.; Milcic, M.; Nikolic, M.; Cirkovic Velickovic, T. Characterization and effects of binding of food-derived bioactive phycocyanobilin to bovine serum albumin. Food Chem. 2018, 239, 1090–1099. [Google Scholar] [CrossRef]
- Yokoyama, T.; Neya, S.; Tsuneshige, A.; Yonetani, T.; Park, S.-Y.; Tame, J.R.H. R-State Haemoglobin with Low Oxygen Affinity: Crystal Structures of Deoxy Human and Carbonmonoxy Horse Haemoglobin Bound to the Effector Molecule L35. J. Mol. Biol. 2006, 356, 790–801. [Google Scholar] [CrossRef]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable Molecular Dynamics on CPU and GPU Architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The VEGA Suite of Programs: An Versatile Platform for Cheminformatics and Drug Design Projects. Bioinformatics 2021, 37, 1174–1175. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC: A Semiempirical Molecular Orbital Program. J. Comput. Aided Mol. Des. 1990, 4, 1–105. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of fluorescence by oxygen. A probe for structural fluctuations in macromolecules. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef]
- Bi, S.; Dinga, L.; Tiana, Y.; Songa, D.; Zhou, X.; Liua, X.; Zhanga, H. Investigation of the interaction between flavonoids and human serum albumin. J. Mol. Struct. 2004, 703, 37–45. [Google Scholar] [CrossRef]
- Pace, N.C.; Scholtz, M.J.; Grimsley, G.R. Forces Stabilizing Proteins. FEBS Lett. 2014, 588, 2177–2184. [Google Scholar] [CrossRef]






| Ligand | T (K) | KSV (M−1) | R2 | Kq (M−1 s−1) |
|---|---|---|---|---|
| 298.15 | 2.22 × 103 | 0.9958 | 2.22 × 1011 | |
| Clozapine | 303.15 | 2.01 × 103 | 0.9957 | 2.01 × 1011 |
| 310.15 | 1.84 × 103 | 0.9952 | 1.84 × 1011 | |
| 298.15 | 0.67 × 103 | 0.9956 | 0.67 × 1011 | |
| Ziprasidone | 303.15 | 0.62 × 103 | 0.9957 | 0.62 × 1011 |
| 310.15 | 0.55 × 103 | 0.9970 | 0.55 × 1011 | |
| 298.15 | 2.71 × 103 | 0.9981 | 2.71 × 1011 | |
| Sertindole | 303.15 | 2.29 × 103 | 0.9957 | 2.29 × 1011 |
| 310.15 | 1.16 × 103 | 0.9911 | 1.16 × 1011 |
| Ligand | T (K) | Ka × 104 (M−1) | n | ΔG (kJ mol−1) | ΔS (J mol−1 K−1) | ΔH (kJ mol−1) |
|---|---|---|---|---|---|---|
| 298.15 | 2.19 | 1.12 | −12.2 | |||
| Clozapine | 303.15 | 2.03 | 1.03 | −12.4 | +41.0 | −12.5 |
| 310.15 | 1.80 | 0.97 | −12.7 | |||
| 298.15 | 0.74 | 1.16 | −12.3 | |||
| Ziprasidone | 303.15 | 0.69 | 1.08 | −12.5 | +41.2 | −9.8 |
| 310.15 | 0.63 | 1.06 | −12.8 | |||
| 298.15 | 1.81 | 1.10 | −16.9 | |||
| Sertindole | 303.15 | 1.71 | 1.06 | −17.2 | +56.8 | −7.4 |
| 310.15 | 1.61 | 1.03 | −17.6 |
| Sample & Monitored Parameter | α-Helices Content (%) | metHHb Content (%) | Protection from Oxidation (AU) |
|---|---|---|---|
| Control HHb (4 µM) | 48.2 ± 0.7 | 3.96 ± 0.20 | 1 (reference) |
| +clozapine (10 µM) | 52.2 ± 0.5 a | 3.86 ± 0.23 | 118 ± 4.1 |
| +ziprasidone (10 µM) | 50.2 ± 0.6 | 5.32 ± 0.27 c | 78 ± 3.2 |
| +sertindole (10 µM) | 49.9 ± 1.0 | 5.14 ± 0.42 b | 60 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platanić Arizanović, L.; Gligorijević, N.; Cvijetić, I.; Mijatović, A.; Ristivojević, M.K.; Minić, S.; Kokić, A.N.; Miljević, Č.; Nikolić, M. Human Hemoglobin and Antipsychotics Clozapine, Ziprasidone and Sertindole: Friends or Foes? Int. J. Mol. Sci. 2023, 24, 8921. https://doi.org/10.3390/ijms24108921
Platanić Arizanović L, Gligorijević N, Cvijetić I, Mijatović A, Ristivojević MK, Minić S, Kokić AN, Miljević Č, Nikolić M. Human Hemoglobin and Antipsychotics Clozapine, Ziprasidone and Sertindole: Friends or Foes? International Journal of Molecular Sciences. 2023; 24(10):8921. https://doi.org/10.3390/ijms24108921
Chicago/Turabian StylePlatanić Arizanović, Lena, Nikola Gligorijević, Ilija Cvijetić, Aleksandar Mijatović, Maja Krstić Ristivojević, Simeon Minić, Aleksandra Nikolić Kokić, Čedo Miljević, and Milan Nikolić. 2023. "Human Hemoglobin and Antipsychotics Clozapine, Ziprasidone and Sertindole: Friends or Foes?" International Journal of Molecular Sciences 24, no. 10: 8921. https://doi.org/10.3390/ijms24108921
APA StylePlatanić Arizanović, L., Gligorijević, N., Cvijetić, I., Mijatović, A., Ristivojević, M. K., Minić, S., Kokić, A. N., Miljević, Č., & Nikolić, M. (2023). Human Hemoglobin and Antipsychotics Clozapine, Ziprasidone and Sertindole: Friends or Foes? International Journal of Molecular Sciences, 24(10), 8921. https://doi.org/10.3390/ijms24108921