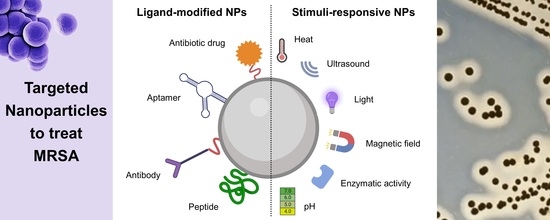
Fighting Methicillin-Resistant Staphylococcus aureus with Targeted Nanoparticles
Abstract
:1. Introduction
2. Properties of Nanoparticles for the Treatment of Bacterial Infections
3. Targeted Nanoparticles for MRSA Therapy
3.1. Metallic Nanoparticles
3.1.1. Gold Nanoparticles
3.1.2. Silver Nanoparticles
3.1.3. Magnetite Nanoparticles
3.1.4. Zinc Nanoparticles
3.2. Polymeric Nanoparticles
3.2.1. PLGA Nanoparticles
3.2.2. Other Polymeric Nanoparticles
3.3. Lipid Nanoparticles
3.3.1. Liposomes
3.3.2. Solid Lipid Nanoparticles
3.3.3. Lipid–Polymer Hybrid Nanoparticles
3.4. Mesoporous Silica Nanoparticles
4. Discussion and Concluding Remarks
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Action Plan on Antimicrobial Resistance; WHO Report; WHO: Geneva, Switzerland, 2015.
- Hibbitts, A.; O’Leary, C. Emerging Nanomedicine Therapies to Counter the Rise of Methicillin-Resistant Staphylococcus aureus. Materials 2018, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Raisen, C.L.; Ba, X.; Sadgrove, N.J.; Padilla-González, G.F.; Simmonds, M.S.J.; Loncaric, I.; Kerschner, H.; Apfalter, P.; Hartl, R.; et al. Emergence of methicillin resistance predates the clinical use of antibiotics. Nature 2022, 602, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, Y.; Cao, Y.; Mo, A.; Peng, Q. Potentials of nanotechnology in treatment of methicillin-resistant Staphylococcus aureus. Eur. J. Med. Chem. 2021, 213, 113056. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Numan, A.; Somaily, H.H.; Gorain, B.; Ranjan, S.; Rilla, K.; Siddique, H.R.; Kesharwani, P. Nano-enabled strategies to combat methicillin-resistant Staphylococcus aureus. Mater. Sci. Eng. C 2021, 129, 112384. [Google Scholar] [CrossRef]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7, 1–23. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Huang, R.; Cai, G.-Q.; Li, J.; Li, X.-S.; Liu, H.-T.; Shang, X.-L.; Zhou, J.-D.; Nie, X.-M.; Gui, R. Platelet membrane-camouflaged silver metal-organic framework drug system against infections caused by methicillin-resistant Staphylococcus aureus. J. Nanobiotechnol. 2021, 19, 229. [Google Scholar] [CrossRef]
- Labruère, R.; Sona, A.; Turos, E. Anti–methicillin-resistant Staphylococcus aureus nanoantibiotics. Front. Pharmacol. 2019, 10, 1121. [Google Scholar] [CrossRef]
- Hulme, J. Application of nanomaterials in the prevention, detection, and treatment of Methicillin-Resistant Staphylococcus aureus (MRSA). Pharmaceutics 2022, 14, 805. [Google Scholar] [CrossRef]
- Dey, N.; Kamatchi, C.; Vickram, A.S.; Anbarasu, K.; Thanigaivel, S.; Palanivelu, J.; Pugazhendhi, A.; Ponnusamy, V.K. Role of nanomaterials in deactivating multiple drug resistance efflux pumps—A review. Environ. Res. 2022, 204, 111968. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Alabdali, A. Application of nanoantibiotics approach against anti-bacterial resistance. Int. J. Appl. Pharm. 2022, 14, 34–39. [Google Scholar] [CrossRef]
- Kirui, D.K.; Weber, G.; Talackine, J.; Millenbaugh, N.J. Targeted laser therapy synergistically enhances efficacy of antibiotics against multi-drug resistant Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102018. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Maji, R.; Omolo, C.A.; Agrawal, N.; Maduray, K.; Hassan, D.; Mokhtar, C.; Mackhraj, I.; Govender, T. pH-Responsive Lipid–Dendrimer Hybrid Nanoparticles: An Approach To Target and Eliminate Intracellular Pathogens. Mol. Pharm. 2019, 16, 4594–4609. [Google Scholar] [CrossRef]
- Thomas-Moore, B.A.; del Valle, C.A.; Field, R.A.; Marín, M.J. Recent advances in nanoparticle-based targeting tactics for antibacterial photodynamic therapy. Photochem. Photobiol. Sci. 2022, 21, 1111–1131. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Li, B.; Chen, D.; Dong, X.; Wang, Y.; Gu, Y. Versatile antimicrobial peptide-based ZnO quantum dots for in vivo bacteria diagnosis and treatment with high specificity. Biomaterials 2015, 53, 532–544. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef]
- Liu, M.; Fang, X.; Yang, Y.; Wang, C. Peptide-Enabled Targeted Delivery Systems for Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 701504. [Google Scholar] [CrossRef]
- Song, K.-M.; Lee, S.; Ban, C. Aptamers and their biological applications. Sensors 2012, 12, 612–631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Sikwal, D.R.; Rambharose, S.; Mocktar, C.; Singh, S.; Bester, L.; Oh, J.K.; Renukuntla, J.; Govender, T. Enhancing targeted antibiotic therapy via pH responsive solid lipid nanoparticles from an acid cleavable lipid. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-Responsive Nanomaterials for Application in Antitumor Therapy and Drug Delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Millenbaugh, N.J.; Baskin, J.B.; DeSilva, M.N.; Elliott, W.R.; Glickman, R.D. Photothermal killing of Staphylococcus aureus using antibody-targeted gold nanoparticles. Int. J. Nanomed. 2015, 10, 1953–1960. [Google Scholar] [CrossRef]
- Ocsoy, I.; Yusufbeyoglu, S.; Yılmaz, V.; McLamore, E.S.; Ildız, N.; Ülgen, A. DNA aptamer functionalized gold nanostructures for molecular recognition and photothermal inactivation of methicillin-Resistant Staphylococcus aureus. Colloids Surf. B 2017, 159, 16–22. [Google Scholar] [CrossRef]
- Wang, H.; Song, Z.; Li, S.; Wu, Y.; Han, H. One Stone with Two Birds: Functional Gold Nanostar for Targeted Combination Therapy of Drug-Resistant Staphylococcus aureus Infection. ACS Appl. Mater. Interfaces 2019, 11, 32659–32669. [Google Scholar] [CrossRef]
- Hu, D.; Li, H.; Wang, B.; Ye, Z.; Lei, W.; Jia, F.; Jin, Q.; Ren, K.-F.; Ji, J. Surface-adaptive gold nanoparticles with effective adherence and enhanced photothermal ablation of methicillin-resistant Staphylococcus aureus biofilm. ACS Nano 2017, 11, 9330–9339. [Google Scholar] [CrossRef]
- Liu, M.; He, D.; Yang, T.; Liu, W.; Mao, L.; Zhu, Y.; Wu, J.; Luo, G.; Deng, J. An efficient antimicrobial depot for infectious site-targeted chemo-photothermal therapy. J. Nanobiotechnol. 2018, 16, 23. [Google Scholar] [CrossRef]
- Karaagac, Z.; Yusufbeyoglu, S.; Ildiz, N.; Sellami, H.; Ocsoy, I. A facile and one-pot aqueous phase transfer of oleylamine capped Au NP with aminophenylboronic acid used as transfer and targeting ligand. Enzyme Microb. 2021, 148, 109810. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Wang, S.-G.; Wu, C.-Y.; Lee, K.-C.; Jao, C.-J.; Chou, S.-H.; Chen, Y.-C. Functional gold nanoparticle-based antibacterial agents for nosocomial and antibiotic-resistant bacteria. Nanomedicine 2016, 11, 2497–2510. [Google Scholar] [CrossRef]
- Meeker, D.G.; Jenkins, S.V.; Miller, E.K.; Beenken, K.E.; Loughran, A.J.; Powless, A.; Muldoon, T.J.; Galanzha, E.I.; Zharov, V.P.; Smeltzer, M.S.; et al. Synergistic Photothermal and Antibiotic Killing of Biofilm-Associated Staphylococcus aureus Using Targeted Antibiotic-Loaded Gold Nanoconstructs. ACS Infect. Dis. 2016, 2, 241–250. [Google Scholar] [CrossRef]
- Meeker, D.G.; Wang, T.; Harrington, W.N.; Zharov, V.P.; Johnson, S.A.; Jenkins, S.V.; Oyibo, S.E.; Walker, C.M.; Mills, W.B.; Shirtliff, M.E. Versatility of targeted antibiotic-loaded gold nanoconstructs for the treatment of biofilm-associated bacterial infections. Int. J. Hyperth. 2018, 34, 209–219. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Yu, Y.; Li, W.; Ma, Z.; Wang, J.; Zhang, X.; Gao, H.; Liu, D. Photoactive silver nanoagents for backgroundless monitoring and precision killing of multidrug-resistant bacteria. Nanotheranostics 2021, 5, 472. [Google Scholar] [CrossRef]
- Zuo, Y.-M.; Yan, X.; Xue, J.; Guo, L.-Y.; Fang, W.-W.; Sun, T.-C.; Li, M.; Zha, Z.; Yu, Q.; Wang, Y. Enzyme-responsive Ag nanoparticle assemblies in targeting antibacterial against methicillin-resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 2020, 12, 4333–4342. [Google Scholar] [CrossRef]
- Huo, D.; Ding, J.; Cui, Y.X.; Xia, L.Y.; Li, H.; He, J.; Zhou, Z.Y.; Wang, H.W.; Hu, Y. X-ray CT and pneumonia inhibition properties of gold–silver nanoparticles for targeting MRSA induced pneumonia. Biomaterials 2014, 35, 7032–7041. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Sun, L.; Zhang, H.; Guo, Y.; Qu, J.; Jiang, W.; Chen, W.; Ji, J.; Yang, Y.W. Synergistic Chemotherapy and Photodynamic Therapy of Endophthalmitis Mediated by Zeolitic Imidazolate Framework-Based Drug Delivery Systems. Small 2019, 15, 1903880. [Google Scholar] [CrossRef]
- Wang, K.-K.; Shin, E.P.; Lee, H.-J.; Jung, S.-J.; Hwang, J.-W.; Heo, I.; Kim, J.-H.; Oh, M.-K.; Kim, Y.-R. Target-oriented photofunctional nanoparticles (TOPFNs) for selective photodynamic inactivation of Methicillin-resistant Staphylococcus aureus (MRSA). J. Photochem. Photobiol. B Biol. 2018, 183, 184–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; Wang, D.; Xu, C.; Sheng, S.; Cheng, J.; Bao, C.; Li, Y.; Tian, H. Fe-TCPP@CS nanoparticles as photodynamic and photothermal agents for efficient antimicrobial therapy. Biomater. Sci. 2020, 8, 6526–6532. [Google Scholar] [CrossRef]
- Chen, W.J.; Tsai, P.J.; Chen, Y.C. Functional Fe3O4/TiO2 core/shell magnetic nanoparticles as photokilling agents for pathogenic bacteria. Small 2008, 4, 485–491. [Google Scholar] [CrossRef]
- A Ocsoy, M.; Yusufbeyoglu, S.; Ildiz, N.; Ulgen, A.; Ocsoy, I. DNA Aptamer-Conjugated Magnetic Graphene Oxide for Pathogenic Bacteria Aggregation: Selective and Enhanced Photothermal Therapy for Effective and Rapid Killing. ACS Omega 2021, 6, 20637–20643. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-X.; Wang, B.-B.; Zhao, X.; Chen, L.-J.; Yan, X.-P. A pH-Responsive Persistent Luminescence Nanozyme for Selective Imaging and Killing of Helicobacter pylori and Common Resistant Bacteria. ACS Appl. Mater. Interfaces 2021, 13, 60955–60965. [Google Scholar] [CrossRef] [PubMed]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Tang, K.; Liao, H.; Xu, Y.; Wang, L.; Niu, C. Multi-Modal Imaging Monitored M2 Macrophage Targeting Sono-Responsive Nanoparticles to Combat MRSA Deep Infections. Int. J. Nanomed. 2022, 17, 4525–4546. [Google Scholar] [CrossRef] [PubMed]
- Ucak, S.; Sudagidan, M.; Borsa, B.A.; Mansuroglu, B.; Ozalp, V.C. Inhibitory effects of aptamer targeted teicoplanin encapsulated PLGA nanoparticles for Staphylococcus aureus strains. World J. Microbiol. Biotechnol. 2020, 36, 69. [Google Scholar] [CrossRef]
- Hu, D.; Zou, L.; Li, B.; Hu, M.; Ye, W.; Ji, J. Photothermal Killing of Methicillin-Resistant Staphylococcus aureus by Bacteria-Targeted Polydopamine Nanoparticles with Nano-Localized Hyperpyrexia. ACS Biomater. Sci. Eng. 2019, 5, 5169–5179. [Google Scholar] [CrossRef]
- Kim, C.-J.; Si, Z.; Reghu, S.; Guo, Z.; Zhang, K.; Li, J.; Chan-Park, M.B. DNA-derived nanostructures selectively capture gram-positive bacteria. Drug Deliv. Transl. Res. 2021, 11, 1438–1450. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. AB2-type amphiphilic block copolymer containing a pH-cleavable hydrazone linkage for targeted antibiotic delivery. Int. J. Pharm. 2020, 575, 118948. [Google Scholar] [CrossRef]
- Guo, X.; Cao, B.; Wang, C.; Lu, S.; Hu, X. In vivo photothermal inhibition of methicillin-resistant Staphylococcus aureus infection by in situ templated formulation of pathogen-targeting phototheranostics. Nanoscale 2020, 12, 7651–7659. [Google Scholar] [CrossRef]
- Wong, E.H.H.; Khin, M.M.; Ravikumar, V.; Si, Z.; Rice, S.A.; Chan-Park, M.B. Modulating Antimicrobial Activity and Mammalian Cell Biocompatibility with Glucosamine-Functionalized Star Polymers. Biomacromolecules 2016, 17, 1170–1178. [Google Scholar] [CrossRef]
- Vanamala, K.; Bhise, K.; Sanchez, H.; Kebriaei, R.; Luong, D.; Sau, S.; Abdelhady, H.; Rybak, M.J.; Andes, D.; Iyer, A.K. Folate functionalized lipid nanoparticles for targeted therapy of methicillin-resistant Staphylococcus aureus. Pharmaceutics 2021, 13, 1791. [Google Scholar] [CrossRef]
- Pang, X.; Xiao, Q.; Cheng, Y.; Ren, E.; Lian, L.; Zhang, Y.; Gao, H.; Wang, X.; Leung, W.; Chen, X. Bacteria-responsive nanoliposomes as smart sonotheranostics for multidrug resistant bacterial infections. ACS Nano 2019, 13, 2427–2438. [Google Scholar] [CrossRef]
- Omolo, C.A.; Hassan, D.; Devnarain, N.; Jaglal, Y.; Mocktar, C.; Kalhapure, R.S.; Jadhav, M.; Govender, T. Formulation of pH responsive multilamellar vesicles for targeted delivery of hydrophilic antibiotics. Colloids Surf. B 2021, 207, 112043. [Google Scholar] [CrossRef]
- Ibrahim, U.H.; Devnarain, N.; Omolo, C.A.; Mocktar, C.; Govender, T. Biomimetic pH/lipase dual responsive vitamin-based solid lipid nanoparticles for on-demand delivery of vancomycin. Int. J. Pharm. 2021, 607, 120960. [Google Scholar] [CrossRef]
- Ghanbar, S.; Fumakia, M.; Ho, E.A.; Liu, S. A new strategy for battling bacterial resistance: Turning potent, non-selective and potentially non-resistance-inducing biocides into selective ones. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 471–481. [Google Scholar] [CrossRef]
- Makhathini, S.S.; Omolo, C.A.; Gannimani, R.; Mocktar, C.; Govender, T. pH-responsive micelles from an oleic acid tail and propionic acid heads dendritic amphiphile for the delivery of antibiotics. J. Pharm. Sci. 2020, 109, 2594–2606. [Google Scholar] [CrossRef]
- Jaglal, Y.; Osman, N.; Omolo, C.A.; Mocktar, C.; Devnarain, N.; Govender, T. Formulation of pH-responsive lipid-polymer hybrid nanoparticles for co-delivery and enhancement of the antibacterial activity of vancomycin and 18β-glycyrrhetinic acid. J. Drug Deliv. Sci. Technol. 2021, 64, 102607. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Niculescu, V.-C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef]
- Nie, B.e.; Huo, S.; Qu, X.; Guo, J.; Liu, X.; Hong, Q.; Wang, Y.; Yang, J.; Yue, B. Bone infection site targeting nanoparticle-antibiotics delivery vehicle to enhance treatment efficacy of orthopedic implant related infection. Bioact. Mater. 2022, 16, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, H.; Lu, X.; Xu, H.; Zhang, H. Targeting Detection and Inhibition of Methicillin-Resistant Staphylococcus aureus Pneumonia with a Theranostic Mesoporous Silica-Based Platform. J. Biomed. Nanotechnol. 2018, 14, 1298–1307. [Google Scholar] [CrossRef]
- Fulaz, S.; Devlin, H.; Vitale, S.; Quinn, L.; O’Gara, J.P.; Casey, E. Tailoring Nanoparticle-Biofilm Interactions to Increase the Efficacy of Antimicrobial Agents Against Staphylococcus aureus. Int. J. Nanomed. 2020, 15, 4779–4791. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Huang, X.; Song, X.; Wu, Y.; Ma, X.; Shen, J.; Zhu, K. A Rigid Nanoplatform for Precise and Responsive Treatment of Intracellular Multidrug-Resistant Bacteria. Engineering 2022, 15, 57–66. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, F.; Guo, G.; Wang, E.; Younis, M.R.; Zhang, Z.; Zhang, F.; Huan, Z.; Fan, C.; Yang, C.; et al. Targeted hot ion therapy of infected wound by glycol chitosan and polydopamine grafted Cu-SiO2 nanoparticles. Nano Today 2021, 41, 101330. [Google Scholar] [CrossRef]
- Devlin, H.; Fulaz, S.; Hiebner, D.W.; O’Gara, J.P.; Casey, E. Enzyme-functionalized mesoporous silica nanoparticles to target Staphylococcus aureus and disperse biofilms. Int. J. Nanomed. 2021, 16, 1929. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef]
- Yeo, W.W.Y.; Maran, S.; Kong, A.S.-Y.; Cheng, W.-H.; Lim, S.-H.E.; Loh, J.-Y.; Lai, K.-S. A Metal-Containing NP Approach to Treat Methicillin-Resistant Staphylococcus aureus (MRSA): Prospects and Challenges. Materials 2022, 15, 5802. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Lipman, N.S.; Jackson, L.R.; Trudel, L.J.; Weis-Garcia, F. Monoclonal Versus Polyclonal Antibodies: Distinguishing Characteristics, Applications, and Information Resources. ILAR J. 2005, 46, 258–268. [Google Scholar] [CrossRef]
- Li, D.; Tang, G.; Yao, H.; Zhu, Y.; Shi, C.; Fu, Q.; Yang, F.; Wang, X. Formulation of pH-responsive PEGylated nanoparticles with high drug loading capacity and programmable drug release for enhanced antibacterial activity. Bioact. Mater. 2022, 16, 47–56. [Google Scholar] [CrossRef]
- Piasentin, N.; Milotti, E.; Chignola, R. The control of acidity in tumor cells: A biophysical model. Sci. Rep. 2020, 10, 13613. [Google Scholar] [CrossRef]
- Mohapatra, A.; Uthaman, S.; Park, I.-K. External and internal stimuli-responsive metallic nanotherapeutics for enhanced anticancer therapy. Front. Mol. Biosci. 2021, 7, 597634. [Google Scholar] [CrossRef]
- Boudet, A.; Sorlin, P.; Pouget, C.; Chiron, R.; Lavigne, J.-P.; Dunyach-Remy, C.; Marchandin, H. Biofilm Formation in Methicillin-Resistant Staphylococcus aureus Isolated in Cystic Fibrosis Patients Is Strain-Dependent and Differentially Influenced by Antibiotics. Front. Microbiol. 2021, 12, 750489. [Google Scholar] [CrossRef]
- Hommes, J.W.; Surewaard, B.G. Intracellular habitation of Staphylococcus aureus: Molecular mechanisms and prospects for antimicrobial therapy. Biomedicines 2022, 10, 1804. [Google Scholar] [CrossRef]
- Surewaard, B.G.; Deniset, J.F.; Zemp, F.J.; Amrein, M.; Otto, M.; Conly, J.; Omri, A.; Yates, R.M.; Kubes, P. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J. Exp. Med. 2016, 213, 1141–1151. [Google Scholar] [CrossRef]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Hiroshi Morisaki, J. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527, 323–328. [Google Scholar] [CrossRef]
- Elsawy, S.; Elsherif, W.M.; Hamed, R. Effect of silver nanoparticles on vancomycin resistant Staphylococcus aureus infection in critically ill patients. Pathog. Glob. Health 2021, 115, 315–324. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef]





| Ligand | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Antibodies | High specificity, long half-life, easily mass-produced | Difficulty of synthesizing high-quality antibodies, high cost, high molecular weight | [19] |
| Aptamers | High affinity/specificity, stability, and reproducibility; small size; easy modification and immobilization | Degradation in biological media, cross reactivity | [22] |
| Peptides | Higher cost-effectiveness than antibodies, small size, high binding efficiency, low metabolic consequences, decreased immunogenicity, easily mass-produced | Low target affinity, high clearance, poor pharmacokinetics, metabolic instability | [21] |
| Antibiotic drugs | Low molecular weight, simplicity of their conjugation, easily produced | Weak interaction with their target | [23] |
| Type of NP | Targeting Ligand | Coating | Therapeutic Agent | Main Conclusions | Development Phase | Ref. |
|---|---|---|---|---|---|---|
| Au NPs | Anti-S. aureus peptidoglycan antibody | n.a. | n.a. | MRSA survival decreased to 58% | In vitro | [26] |
| Au NPs | Anti-S. aureus peptidoglycan antibody | n.a. | n.a. | 96% of the MRSA biofilm was removed; NPs conjugation increased MRSA biofilm binding by 7-fold compared to non-conjugated NPs | In vitro | [14] |
| Au NPs and NRs | DNA aptamer | n.a. | n.a. | Both Au nanocarriers accumulated in the MRSA surface and not in the surface of control bacteria; Au NRs inactivated over 95% of cells; no effect for Au NPs | In vitro | [27] |
| Au NPs | Vancomycin | n.a. | n.a. | Vancomycin-NPs showed targeting ability towards MRSA; NPs improved wound healing in vivo; biocompatible | In vivo | [28] |
| Au NPs | (10-mercaptodecyl)trimethylammonium bromide and 11-mercaptoundecanoic acid | n.a. | n.a. | NPs decreased the number of living bacteria without damaging the healthy tissues around the biofilm | In vivo | [29] |
| Au NRs | Glycol chitosan | PDA | n.a. | NRs were observed at the inflammatory site but not in the normal skin; treated mice showed no inflammation or abscess; no damage in the surrounding healthy tissues | In vivo | [30] |
| Au NPs | 3-APBA | OLA | n.a. | 3-APBA binds specifically to the MRSA membrane | In vitro | [31] |
| Au NPs | DVFLG peptide modified with arginine and tryptophan | n.a. | n.a. | NPs slowed down the growth of MRSA in a concentration-dependent manner; low toxicity toward non-target cells | In vitro | [32] |
| Au nanocages | Staphylococcal protein A antibody | PDA | Daptomycin | Conjugated nanocages killed significantly more MRSA than unconjugated nanocages; lack of binding of conjugated nanocages to mammalian cells and S. epidermidis | In vitro | [33] |
| Au nanocages | aLpp and aMntC antibodies | PDA | Daptomycin | Targeting ability of antibody-conjugated nanocarriers compared to unconjugated nanocages; in vitro antibiotic activity against MRSA | In vitro | [34] |
| Ag NPs | Poly[4-O-(α-D-glucopyranosyl)-D-glucopyranose] | n.a. | Chlorin e6 | Bacteria survival of 3% in MRSA-infected mice, resulting in accelerated wound repair; biocompatible | In vivo | [35] |
| Ag NPs | Enzyme-responsive branch polymers | n.a. | n.a. | Enhanced MRSA killing rate of ANAs, resulting in accelerated healing of MRSA infections; biocompatible | In vivo | [36] |
| Au–Ag NPs | Anti-MRSA antibody | n.a. | n.a. | Compared to unconjugated NPs, antibody-modified NPs showed an 11-fold enhancement in targeting MRSA in vitro; reduction in the inflammation in vivo; biocompatible | In vivo | [37] |
| Ag NPs | Platelet membrane | n.a. | Vancomycin | Vancomycin-loaded modified NPs exhibited a greater ability to inhibit MRSA growth than unmodified loaded NPs and free vancomycin; biocompatible | In vivo | [8] |
| Ag NPs | Vancomycin | n.a. | Ammonium methylbenzene blue | Increased biofilm eradication after NP functionalization; biocompatible | In vivo | [38] |
| Magnetite NPs | MRSA antibody | n.a. | n.a. | NPs showed selective killing ability for MRSA with minimum damage to mouse fibroblast cells; MRSA infection rate of mice with skin infection decreased to 38% | In vivo | [39] |
| Magnetite NPs | Chitosan | n.a. | n.a. | Decrease in MRSA colonies by 98% | In vitro | [40] |
| Magnetite NPs | IgG antibody | Titania | n.a. | Ability to target MRSA | In vitro | [41] |
| Magnetite NPs | DNA aptamer | n.a. | n.a. | Targeted NPs exhibited higher cell inactivation activity compared to non-targeted NPs | In vitro | [42] |
| ZnO quantum dots | UBI29-41 peptide | n.a. | Methicillin | UBI29-4 improved MRSA specificity; combining methicillin and modified NPs improved their individual anti-MRSA properties | In vivo | [18] |
| Zinc gallogermanate NPs | Chitosan-benzeneboronic acid | Mesoporous silica | n.a. | Surface modification allowed for the presence of the NPs in the inflammatory region; abscesses and inflammation on the skin of mice treated with targeted NPs disappeared but remained in the non-targeted NP group | In vivo | [43] |
| NP Composition | Targeting Ligand | Therapeutic Agent | Main Conclusions | Development Phase | Ref. |
|---|---|---|---|---|---|
| PLGA NPs | M2 macrophage membrane | IR780 | The coating increased NP accumulation at the infection site, improving the antibacterial efficacy of the DDS; biocompatible | In vivo | [45] |
| PLGA NPs | Aptamer | Teicoplanin | Targeting capacity of the DDS to S. aureus over S. epidermidis cells; functionalization led to a 32-fold decrease in MIC compared to non-functionalized NPs | In vitro | [46] |
| PDA NPs | Vancomycin | n.a. | Vancomycin enhanced NP adhesion to the MRSA surface; NPs rapidly targeted the MRSA-infected site; biocompatible | In vivo | [47] |
| Polystyrene- DNA strand micelles | n.a. | n.a. | NPs efficiently selected Gram-positive strains over Gram-negative strains; over 90% of MRSA strains were captured | In vitro | [48] |
| AB2-type amphiphilic micelles | n.a. | Vancomycin | pH-dependent drug release resulted in enhanced in vitro antibacterial activity of vancomycin at basic pH; vancomycin-loaded NPs showed superior ability to treat MRSA infections relative to free drug | In vivo | [49] |
| Polypyrrole NPs | Vancomycin | n.a. | Modified NPs showed a higher ability to inhibit MRSA infection than non-modified NPs; biocompatible | In vivo | [50] |
| Polylysine glycopolymer stars | Glucosamine | n.a. | The antimicrobial efficacy of NPs was selective toward Gram-positive bacteria, including MRSA; biocompatible | In vitro | [51] |
| Type of NP | Targeting Ligand | Therapeutic Agent | Main Conclusions | Development Phase | Ref. |
|---|---|---|---|---|---|
| Liposomes | Folate | Vancomycin | Compared to free vancomycin, folate-decorated NPs showed enhanced accumulation in MRSA-infected tissues, resulting in a higher bactericidal effect and reduced accumulation in kidneys and liver | In vivo | [52] |
| Liposomes | Maltohexaose | Purpurin 18 | Modified NPs targeted the infection site; specificity for MRSA-infected sites and not inflammation sites and cancer; effective MRSA killing; biocompatible | In vivo | [53] |
| SLNs | Oleic acid and stearyl amine | Vancomycin | Higher anti-MRSA activity of vancomycin-loaded SLNs compared to free vancomycin | In vivo | [54] |
| SLNs | SA-3M | Vancomycin | Vancomycin-loaded SLNs led to a 22-fold decrease in MRSA survival compared to free vancomycin | In vivo | [24] |
| SLNs | Ascorbyl tocopherol succinate | Vancomycin | Free vancomycin resulted in a 4-fold reduction in bacterial load of MRSA-infected mice compared to the untreated group, while vancomycin-loaded NPs decreased the bacterial load by 13-fold; biocompatible | In vivo | [55] |
| SLNs | Anti-MRSA antibody (NYR MRSA 16) | C17 | SLNs with anti-MRSA antibodies were more effective against MRSA than unconjugated or IgG-conjugated SLNs; selective toxicity toward MRSA | In vitro | [56] |
| LPHNs | n.a. | Vancomycin | Free vancomycin showed no antimicrobial activity, while vancomycin-loaded LPHNs reduced the MRSA load | In vitro | [16] |
| LPHNs | n.a. | Vancomycin | DDS resulted in an eightfold reduction in the MRSA burden of infected mice compared to the free drug | In vivo | [57] |
| LPHNs | n.a. | Vancomycin + 18β-glycyrrhetinic acid | NPs presented a synergistic effect in terms of elimination of MRSA cells and MRSA biofilm compared to free vancomycin and 18β-glycyrrhetinic acid | In vitro | [58] |
| Therapeutic Agent | Targeting Ligand | Main Conclusions | Development Phase | Ref. |
|---|---|---|---|---|
| Vancomycin | D6 and UBI29-41 peptides | Vancomycin-loaded, dual-targeted NPs showed the largest decrease in bone destruction and preserved bone integrity in vivo; biocompatible | In vivo | [62] |
| Vancomycin | Anti-MRSA antibody | Modified MSNs exhibited a sevenfold higher binding efficacy against MRSA than non-modified NPs, resulting in a higher antiproliferation effect; biocompatible | In vivo | [63] |
| Vancomycin | Amine, carboxyl, and aromatic groups | Positively charged NPs were more efficiently bonded to the MRSA surface, resulting in a higher capacity to reduce biofilm viability | In vitro | [64] |
| Rifampin | Phosphatidylglycerol and phosphatidylcholine | NP modification increased MRSA eradication compared to non-modified NPs, accelerating wound healing; biocompatible | In vivo | [65] |
| Copper | Glycol chitosan | NP functionalization enhanced their accumulation on the infection site; improved wound-healing activity; biocompatible | In vivo | [66] |
| Serrapeptase and DNase I | Lysostaphin | Functionalized NPs showed a greater ability to reduce the viability of MRSA than non-functionalized MSNs | In vitro | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, S.; Ramalho, M.J.; Santos, S.B.; Melo, L.D.R.; Santos, R.S.; Guimarães, N.; Azevedo, N.F.; Loureiro, J.A.; Pereira, M.C. Fighting Methicillin-Resistant Staphylococcus aureus with Targeted Nanoparticles. Int. J. Mol. Sci. 2023, 24, 9030. https://doi.org/10.3390/ijms24109030
Andrade S, Ramalho MJ, Santos SB, Melo LDR, Santos RS, Guimarães N, Azevedo NF, Loureiro JA, Pereira MC. Fighting Methicillin-Resistant Staphylococcus aureus with Targeted Nanoparticles. International Journal of Molecular Sciences. 2023; 24(10):9030. https://doi.org/10.3390/ijms24109030
Chicago/Turabian StyleAndrade, Stéphanie, Maria J. Ramalho, Sílvio B. Santos, Luís D. R. Melo, Rita S. Santos, Nuno Guimarães, Nuno F. Azevedo, Joana A. Loureiro, and Maria C. Pereira. 2023. "Fighting Methicillin-Resistant Staphylococcus aureus with Targeted Nanoparticles" International Journal of Molecular Sciences 24, no. 10: 9030. https://doi.org/10.3390/ijms24109030
APA StyleAndrade, S., Ramalho, M. J., Santos, S. B., Melo, L. D. R., Santos, R. S., Guimarães, N., Azevedo, N. F., Loureiro, J. A., & Pereira, M. C. (2023). Fighting Methicillin-Resistant Staphylococcus aureus with Targeted Nanoparticles. International Journal of Molecular Sciences, 24(10), 9030. https://doi.org/10.3390/ijms24109030