A Microfluidic Experimental Method for Studying Cell-to-Cell Exosome Delivery–Taking Stem Cell–Tumor Cell Interaction as a Case
Abstract
:1. Introduction
2. Results
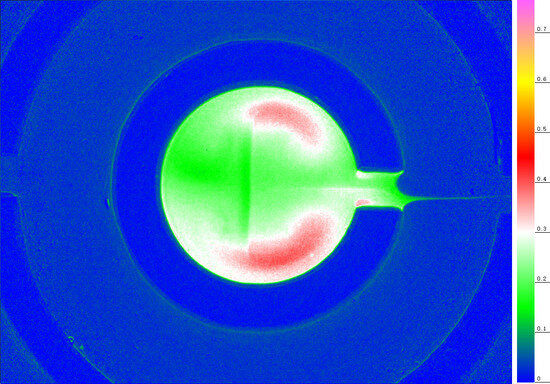
2.1. The Artificial Cell Nest for Transport and Deposition Modes of Nanoparticles
2.2. The Stem-Cell-to-Tumor-Cell Co-Culture without Microfluidics
2.3. The Microfluidic Co-Cultivation of Stem Cells and Tumor Cells in Typical Culture Dishes
2.4. The Interaction between Stem Cells and Tumor Cells in Artificial Cell Nests in a Microfluidic Culture
2.5. A Long-Distance Induction Experiment of Directed Flow in an Artificial Cell Nest
3. Discussion
4. Materials and Methods
4.1. The Fluid Drive Technology
4.2. The Drive of Fluid in an Ordinary Culture Dish
4.3. The Microfluidic Culture Dish
4.4. The Micro Photographing Method of Ultra-Slow Microcirculation
4.5. The Calculation Method of the Flux into the Artificial Cell Nest
4.6. The Cell Growth Method in Matrigel
4.7. The Image Enhancement Method of Cells in Matrigel
4.8. The Extraction Method of Stem Cell Exosomes
4.9. The Cell Lines of Stem Cells and Tumor Cells
5. Conclusions
- (A)
- The extraction, storage, and feeding process includes many uncertain processes, such as denaturation, degradation, concentration change, and pollution.
- (B)
- Nonreal-time live cell exosome delivery cannot reflect the details of cell–cell interaction feedback.
- (C)
- Manual intervention experiments such as cutting off, reversing direction, and program changes cannot be immediately implemented in the process of cell–cell communication.
- (D)
- These experiments consume a lot of time, samples, and related resources.
- (E)
- Hormonal effects between cells of the same type cannot be ruled out during the culture collection of many cells of the same type.
- (F)
- It is inconvenient to conduct real-time drug intervention cell–cell communication experiments (i.e., related drug development experiments).
- (A)
- Cocultivation and real-time delivery of exosomes in situ avoid denaturation, degradation, concentration changes, and contamination.
- (B)
- The exosome interaction of living cells during coculture occurs in situ.
- (C)
- Under the condition of ensuring the correct stem cell microenvironment, the programmed flow direction, flow rate, and flow field can realize multiple real-time manual intervention experiments such as cutting off exosome communication, reversing the direction, and resetting the program.
- (D)
- Eliminates many cultured cells and exosome isolation and purification processes, significantly saving time and cost.
- (E)
- Cocultivation and interaction between single-cell clones or small cell clusters, or even single cells can be achieved with solid pertinence and transparent and reliable information transmission logic between cells.
- (F)
- The online coculture of live cells containing exosome delivery direction control can be further combined with drug testing or experimentation, greatly expanding the drug development technology, and improving the accuracy and speed of drug development.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reagent or Resource | Source | Identifier |
|---|---|---|
| Biological Samples | ||
| A549-RFP cell line | xiangfbio | N/A |
| hiPSC-GFP | cellapybio | N/A |
| hESC-H9-eGFP | cellapybio | N/A |
| human normal liver cell | cellbank | N/A |
| Medium and reagents | ||
| F-12 Medium | Thermo | SH30023.01 |
| 1640 Medium | gibco | N/A |
| PSCeasy hESC/hiPSC Medium | cellapybio | 1001500 |
| Matrigel Matrix | Corning | N/A |
| Magnetic O-pump | www.ufdish.com/, accessed on 6 March 2023. | N/A |
| Microfluidic culture dish | www.ufdish.com/, accessed on 6 March 2023. | N/A |
References
- Nagasawa, R.; Nomura, N.; Obana, N. Identification of a Novel Gene Involved in Cell-to-cell Communication-induced Cell Death and eDNA Production in Streptococcus mutans. Microbes Environ. 2023, 38, 22085. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.; Carmona, M.; Luna, O.; Gómez, F.A.; Cárdenas, C.; Flores-Herrera, P.; Belmonte, R.; Marshall, S.H. Serum-isolated exosomes from Piscirickettsia salmonis-infected Salmo salar specimens enclose bacterial DnaK, DnaJ and GrpE chaperones. Electron. J. Biotechnol. 2022, 59, 83–93. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Xunian, Z.; Kalluri, R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020, 111, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–117. [Google Scholar] [CrossRef] [PubMed]
- Gusachenko, O.N.; Zenkova, M.A.; Vlassov, V.V. Nucleic acids in exosomes: Disease markers and intercellular communication molecules. Biochemistry 2013, 78, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.D.; Rimmer, M.P. Extracellular vesicles arising from apoptosis: Forms, functions, and applications. J. Pathol. 2023, 9, 6138. [Google Scholar] [CrossRef] [PubMed]
- Witsch, J.; Roh, D.S.; Iadecola, C.; Diaz-Arrastia, R.; Kasner, S.E.; Mayer, S.A.; Murthy, S.B. Association Between Soluble Intercellular Adhesion Molecule-1 and Intracerebral Hemorrhage Outcomes in the FAST Trial. Stroke 2023, 25, 042466. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Peng, X.Y.; Peng, L.; Guo, Y.A. Highly Programmable Magnetic Micropump. Adv. Mater. Technol. 2021, 6, 2100150. [Google Scholar] [CrossRef]
- Peng, X.Y.; Guo, Y.; Peng, L.; Liu, J. Design Artificial Stem Cell Nests for Stem Cell Niche in a Microfluidic Petri Dish Programmed by a Cell Phone. Adv. Mater. Technol. 2021, 6, 2100045. [Google Scholar] [CrossRef]
- Peng, X.Y.; Dong, B.; Liu, X. Cancer metastasis is related to normal tissue stemness. PLoS ONE 2022, 17, 0277811. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Sugimoto, H.; O’Conne, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Body, J.J.; Aapro, M.; Hadji, P.; Herrstedt, J. Bone health in cancer Apatients: ESMO Clinical Practice Guidelines. Ann Oncol. ESMO Guidel. Work. Group 2014, 25, 24–37. [Google Scholar] [CrossRef]
- Kudo-Saito, C. Cancer-associated mesenchymal stem cells aggravate tumor progression. Front. Cell Dev. Biol. 2015, 3, 23. [Google Scholar] [CrossRef]
- Roccaro, A.M.; Sacco, A.; Maiso, P.; Azab, A.K.; Tai, Y.T.; Reagan, M.; Azab, F.; Flores, L.M.; Campigotto, F.; Weller, E.; et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Investig. 2013, 123, 1542–1555. [Google Scholar] [CrossRef]
- Vangala, G.; Imhoff, F.M.; Squires, C.M.L.; Cridge, A.G.; Baird, S.K. Mesenchymal stem cell homing towards cancer cells is increased by enzyme activity of cathepsin D. Exp. Cell Res. 2019, 383, 111494. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Hyenne, V.; Ghoroghi, S.; Collot, M.; Bons, J.; Follain, G.; Harlepp, S.; Mary, B.; Bauer, J.; Mercier, L.; Busnelli, I.; et al. Studying the Fate of Tumor Extracellular Vesicles at High Spatiotemporal Resolution Using the Zebrafish Embryo. Dev. Cell 2019, 48, 554–572. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. Blymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Schneider, A.; Simons, M. Exosomes: Vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013, 352, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Small, A.C.; Tsao, C.-K.; Moshier, E.L.; Gartrell, B.A.; Wisnivesky, J.P.; Godbold, J.H.; Smith, C.B.; Sonpavde, G.; Oh, W.K.; Galsky, M.D. Prevalence and characteristics of patients with metastatic cancer who receive no anticancer therapy. Cancer 2012, 118, 5947. [Google Scholar] [CrossRef]
- Russo, J. The Mechanisms of Breast Cancer Metastasis. In The Pathobiology of Breast Cancer; Springer: Berlin/Heidelberg, Germany, 2016; pp. 135–148. [Google Scholar] [CrossRef]
- Leslie, P.L.; Chao, Y.L.; Tsai, Y.-H.; Ghosh, S.K.; Porrello, A.; Van Swearingen, A.E.D.; Harrison, E.B.; Cooley, B.C.; Parker, J.S.; Carey, L.A.; et al. Histone deacetylase 11 inhibition promotes breast cancer metastasis from lymph nodes. Nat. Commun. 2019, 10, 4192. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Goswami, S.; Sahai, E.; Wyckoff, J.B.; Segall, J.E.; Condeelis, J.S. Tumor cells caught in the act of invading: Their strategy for enhanced cell motility. Trends Cell Biol. 2005, 15, 138–145. [Google Scholar] [CrossRef]
- Olempska, M.; Eisenach, P.A.; Ammerpohl, O.; Ungefroren, H.; Fandrich, F.; Kalthoff, H. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat. Dis. Int. 2007, 6, 92–97. [Google Scholar] [PubMed]
- Han, H.W.; Hsu, S.H. Chitosan-hyaluronan based 3D co-culture platform for studying the crosstalk of lung cancer cells and mesenchymal stem cells. Acta Biomater. 2016, 42, 157–167. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Doğan, A.; Demirci, S.; Apdik, H.; Apdik, E.A.; Fikrettin, S. Dental pulp stem cells (DPSCs) increase prostate cancer cell proliferation and migration under in vitro conditions. Tissue Cell 2017, 49, 711–718. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.Y.; Su, P.; Guo, Y.; Zhang, J.; Peng, L.; Zhang, R. A Microfluidic Experimental Method for Studying Cell-to-Cell Exosome Delivery–Taking Stem Cell–Tumor Cell Interaction as a Case. Int. J. Mol. Sci. 2023, 24, 13419. https://doi.org/10.3390/ijms241713419
Peng XY, Su P, Guo Y, Zhang J, Peng L, Zhang R. A Microfluidic Experimental Method for Studying Cell-to-Cell Exosome Delivery–Taking Stem Cell–Tumor Cell Interaction as a Case. International Journal of Molecular Sciences. 2023; 24(17):13419. https://doi.org/10.3390/ijms241713419
Chicago/Turabian StylePeng, Xing Yue (Larry), Pengxiang Su, Yaxin Guo, Jing Zhang, Linghan Peng, and Rongrong Zhang. 2023. "A Microfluidic Experimental Method for Studying Cell-to-Cell Exosome Delivery–Taking Stem Cell–Tumor Cell Interaction as a Case" International Journal of Molecular Sciences 24, no. 17: 13419. https://doi.org/10.3390/ijms241713419
APA StylePeng, X. Y., Su, P., Guo, Y., Zhang, J., Peng, L., & Zhang, R. (2023). A Microfluidic Experimental Method for Studying Cell-to-Cell Exosome Delivery–Taking Stem Cell–Tumor Cell Interaction as a Case. International Journal of Molecular Sciences, 24(17), 13419. https://doi.org/10.3390/ijms241713419