Melatonin Alleviates Intestinal Barrier Damaging Effects Induced by Polyethylene Microplastics in Albino Rats
Abstract
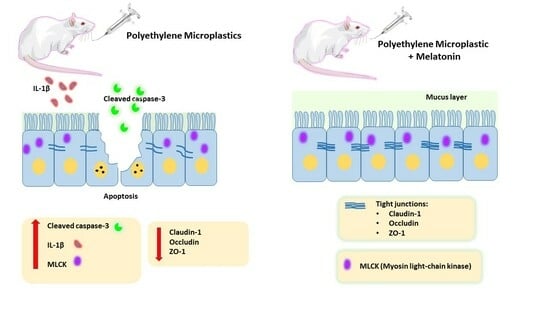
:1. Introduction
2. Results
2.1. PE-MP Particles Uptake by the Jejunum
2.2. Melatonin Improved Jejunal Histopathological Changes Induced by PE-MP Particles
2.3. Melatonin Improved the Enterocytes Ultrastructure Pathological Changes Induced by the PE-MP Particles
2.4. Impact of PE-MP Exposure and Melatonin Treatment on the Mucin Secretion and Muc2 mRNA Expression
2.5. Impact of PE-MP Exposure and Melatonin Treatment on the Expression of Intestinal Epithelial Tight Junction Proteins Occludin, ZO-1, Claudin-1, and MLCK
2.6. Effect of PE-MP Exposure and Melatonin Treatment on the Proinflammatory Cytokines (IL-1β and TNF-α)
2.7. Melatonin Effect on Intestinal Apoptosis Induced by PE-MP Exposure
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Study Design
4.4. Microplastics Quantification
4.5. Biochemical Analysis
4.6. Quantitative Real-Time Polymerase Chain Reaction (qPCR) Analysis for mRNA Gene Expression of MUC2, Occludin, ZO-1, and MLCK
4.6.1. Total RNA Extraction and Reverse Transcription
4.6.2. Quantitative Real-Time PCR
4.7. Histological Study
4.8. Immunohistochemical Study
4.9. Transmission Electron Microscopic Examination
4.10. Imaging
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in environment: Glob-al concern, challenges, and controlling measures. Int. J. Environ. Sci. Technol. 2022, 20, 4673–4694. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wei, Y.; Dong, J.; Zhao, P.; Wang, Y.; Pan, X.; Wang, J. Separation and characterization of microplastic and nanoplastic particles in marine environment. Environ. Pollut. 2022, 297, 118773. [Google Scholar] [CrossRef] [PubMed]
- Kalogerakis, N.; Karkanorachaki, K.; Kalogerakis, G.C.; Triantafyllidi, E.I.; Gotsis, A.D.; Partsinevelos, P.; Fava, F. Microplastics Generation: Onset of Fragmentation of Polyethylene Films in Marine Environment Mesocosms. Front. Mar. Sci. 2017, 4, 84. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of drinking water: A systematic review. PLoS ONE 2020, 15, e0236838. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest wide-spread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Thoo, L.; Noti, M.; Krebs, P. Keep calm: The intestinal barrier at the interface of peace and war. Cell Death Dis. 2019, 10, 849. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between tight junctions & adherens junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [CrossRef]
- Suzuki, T.; Tanabe, S.; Hara, H. Kaempferol Enhances Intestinal Barrier Function through the Cytoskeletal Association and Expression of Tight Junction Proteins in Caco-2 Cells. J. Nutr. 2011, 141, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cao, S.; Xiao, H.; Hu, S.; Yao, K.; Huang, K.; Jiang, Z.; Wang, L. Lactobacillus reuteri 1 Enhances Intestinal Epithelial Barrier Function and Alleviates the Inflammatory Response Induced by Enterotoxigenic Escherichia coli K88 via Suppressing the MLCK Signal-ing Pathway in IPEC-J2 Cells. Front. Immunol. 2022, 13, 897395. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Lu, Y.-Z.; Wu, L.-L.; Yu, L.C. Role of myosin light chain kinase in intestinal epithelial barrier defects in a rat model of bowel obstruction. BMC Gastroenterol. 2010, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and fate of dietary nanoparticles and microparticles in the gastro-intestinal tract. J. Autoimmun. 2010, 34, J226–J233. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Weng, Y.; Shen, Q.; Zhao, Y.; Jin, Y. Microplastic: A potential threat to human and animal health by interfering with the intestinal barrier function and changing the intestinal microenvironment. Sci. Total Environ. 2021, 785, 147365. [Google Scholar] [CrossRef]
- Rawle, D.J.; Dumenil, T.; Tang, B.; Bishop, C.R.; Yan, K.; Le, T.T.; Suhrbier, A. Microplastic consumption induces inflammatory signa-tures in the colon and prolongs a viral arthritis. Sci. Total Environ. 2022, 809, 152212. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Jia, R.; Han, J.; Liu, X.; Li, K.; Lai, W.; Bian, L.; Yan, J.; Xi, Z. Exposure to Polypropylene Microplastics via Oral Ingestion Induces Colon-ic Apoptosis and Intestinal Barrier Damage through Oxidative Stress and Inflammation in Mice. Toxics 2023, 11, 127. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Polyvinyl chloride microplastics induced gut barrier dysfunction, mi-crobiota dysbiosis and metabolism disorder in adult mice. Ecotoxicol. Environ. Saf. 2022, 241, 113809. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, L.; Wu, J.; An, S.; Fang, H.; Han, Y.; Huang, Q.; Chen, Z.; Zeng, Z. Melatonin Attenuates Sepsis-Induced Small-Intestine Injury by Upregulat-ing SIRT3-Mediated Oxidative-Stress Inhibition, Mitochondrial Protection, and Autophagy Induction. Front. Immunol. 2021, 12, 625627. [Google Scholar] [CrossRef] [PubMed]
- Vural, H.; Sabuncu, T.; Arslan, S.O.; Aksoy, N. Melatonin inhibits lipid peroxidation and stimulates the antioxidant status of diabetic rats. J. Pineal Res. 2001, 31, 193–198. [Google Scholar] [CrossRef]
- Maestroni, G.J. The immunotherapeutic potential of melatonin. Expert. Opin. Investig. Drugs 2001, 10, 467–476. [Google Scholar] [CrossRef]
- Ahmed, O.; Farid, A.; Elamir, A. Dual role of melatonin as an anti-colitis and anti-extra intestinal alterations against acetic ac-id-induced colitis model in rats. Sci. Rep. 2022, 12, 6344. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.; Son, M.; Cheon, J.H.; Park, Y.S. Melatonin controls microbiota in colitis by goblet cell differentiation and antimicrobial peptide production through Toll-like receptor 4 signalling. Sci. Rep. 2020, 10, 2232. [Google Scholar] [CrossRef]
- Siah, K.T.H. Melatonin for the treatment of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 2492. [Google Scholar] [CrossRef]
- Chung, S.H.; Park, Y.S.; Kim, O.S.; Kim, J.H.; Baik, H.W.; Hong, Y.O.; Kim, S.S.; Shin, J.H.; Jun, J.H.; Jo, Y.; et al. Melatonin Attenuates Dextran Sodium Sulfate Induced Colitis with Sleep Deprivation: Possible Mechanism by Microarray Analysis. Dig. Dis. Sci. 2014, 59, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Faghih Dinevari, M.; Jafarzadeh, F.; Jabbaripour Sarmadian, A.; Abbasian, S.; Nikniaz, Z.; Riazi, A. The effect of melatonin on irritable bowel syndrome patients with and without sleep disorders: A randomized double-blinded placebo-controlled trial study. BMC Gastroenterol. 2023, 23, 135. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and inflammation-Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef]
- Sun, H.; Chen, N.; Yang, X.; Xia, Y.; Wu, D. Effects induced by polyethylene microplastics oral exposure on colon mucin re-lease, inflammation, gut microflora composition and metabolism in mice. Ecotoxicol. Environ. Saf. 2021, 220, 112340. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Han, J.S.; Park, E.J.; Seong, E.; Lee, G.H.; Kim, D.W.; Son, H.Y.; Han, H.Y.; Lee, B.S. Repeated-oral dose toxicity of polyethylene microplas-tics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, J.; Sohn, J.; Kim, C. Health Effects of Microplastic Exposures: Current Issues and Perspectives in South Korea. Yonsei Med. J. 2023, 64, 301. [Google Scholar] [CrossRef]
- Schwarzfischer, M.; Rogler, G. The Intestinal Barrier—Shielding the Body from Nano- and Microparticles in Our Diet. Metabo-lites 2022, 12, 223. [Google Scholar] [CrossRef]
- Lee, Y.M.; Park, J.P.; Jung, Y.H.; Lee, H.J.; Kim, J.S.; Choi, G.E.; Han, H.J.; Lee, S.J. Melatonin restores Muc2 depletion induced by V. vulnifi-cus VvpM via melatonin receptor 2 coupling with Gαq. J. Biomed. Sci. 2020, 27, 21. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Stern, A.; Peng, H.-H.; Chen, J.-H.; Yang, H.-C. Redox and Metabolic Regulation of Intestinal Barrier Function and Associated Disorders. Int. J. Mol. Sci. 2022, 23, 14463. [Google Scholar] [CrossRef]
- Toto, B.; Refosco, A.; O’Keeffe, M.; Barkhald, Ø.H.; Brønstad, A.; Lied, G.A.; Yadetie, F.; Goksøyr, A.; Kögel, T.; Dierkes, J. Intestinal permeability and gene expression after polyethylene and polyamide microplastic ingestion in Wistar rats. Toxicol. Lett. 2022, 370, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of gut microbiota plays an important role in mi-cro/nanoplastics-induced gut barrier dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wang, Z.; Cao, J.; Gao, T.; Dong, Y.; Chen, Y. Role of melatonin in intestinal mucosal injury induced by restraint stress in mice. Pharm. Biol. 2020, 58, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.; McLean, M.H.; Durum, S.K. Cytokine Tuning of Intestinal Epithelial Function. Front. Immunol. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Guo, S.; Ye, D.; Ma, T.Y. TNF-α Modulation of Intestinal Epithelial Tight Junction Barrier Is Regulated by ERK1/2 Activation of Elk-1. Am. J. Pathol. 2013, 183, 1871–1884. [Google Scholar] [CrossRef]
- Xu, P.; Elamin, E.; Elizalde, M.; Bours, P.P.H.A.; Pierik, M.J.; Masclee, A.A.M.; Jonkers, D.M.A.E. Modulation of Intestinal Epithelial Permeability by Plasma from Patients with Crohn’s Disease in a Three-dimensional Cell Culture Model. Sci. Rep. 2019, 9, 2030. [Google Scholar] [CrossRef]
- Busch, M.; Bredeck, G.; Kämpfer, A.A.M.; Schins, R.P.F. Investigations of acute effects of polystyrene and polyvinyl chloride micro- and nanoplastics in an advanced in vitro triple culture model of the healthy and inflamed intestine. Environ. Res. 2021, 193, 110536. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef]
- Han, S.; Bang, J.; Choi, D.; Hwang, J.; Kim, T.; Oh, Y.; Hwang, Y.; Choi, J.; Hong, J. Surface Pattern Analysis of Microplastics and Their Impact on Human-Derived Cells. ACS Appl. Polym. Mater. 2020, 2, 4541–4550. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; Wei, X.; Chang, L.; Liu, S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total Environ. 2021, 750, 143085. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Jo, J.; Acharya, M.; Maharjan, A.; Lee, D.; KC, P.B.; Kim, C.; Kim, K.; Kim, H.; Heo, Y. Evaluation of potential toxicity of polyethylene microplastics on human derived cell lines. Sci. Total Environ. 2022, 838, 156089. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.A.; Youssef, H.S.; Sliem, R.E.; El Gazzar, W.B.; Nabil, N.; Mokhtar, M.M.; Marei, Y.M.; Ismail, N.S.; Radwaan, S.E.; Badr, A.M.; et al. Hematological consequences of poly-ethylene microplastics toxicity in male rats: Oxidative stress, genetic, and epigenetic links. Toxicology 2023, 492, 153545. [Google Scholar] [CrossRef] [PubMed]
- Linowiecka, K.; Slominski, A.T.; Reiter, R.J.; Böhm, M.; Steinbrink, K.; Paus, R.; Kleszczyński, K. Melatonin: A Potential Regulator of DNA Methylation. Antioxidants 2023, 12, 1155. [Google Scholar] [CrossRef]
- Song, Z.; Humar, B.; Gupta, A.; Maurizio, E.; Borgeaud, N.; Graf, R.; Clavien, P.A.; Tian, Y. Exogenous melatonin protects small-for-size liver grafts by promoting monocyte infiltration and releases interleukin-6. J. Pineal Res. 2018, 65, e12486. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, J.E.; Lee, S.J.; Gong, J.E.; Jin, Y.J.; Seo, S.; Lee, J.H.; Hwang, D.Y. Inflammatory response in the mid colon of ICR mice treated with polystyrene microplastics for two weeks. Lab. Anim. Res. 2021, 37, 31. [Google Scholar] [CrossRef]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicu-lar toxicity in mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, D.K.; Jeong, J.; Yang, S.I.; Kim, J.S.; Kim, J.; Cho, W.S. The reactive oxygen species as pathogenic factors of frag-mented microplastics to macrophages. Environ. Pollut. 2021, 281, 117006. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Lv, W.; Wang, H.; Chen, H.; Xu, Q.; Cai, H.; Dai, J. Intratracheal administration of polystyrene microplastics induces pulmonary fibrosis by activating oxidative stress and Wnt/β-catenin signaling pathway in mice. Ecotoxicol. Environ. Saf. 2022, 232, 113238. [Google Scholar] [CrossRef]
- Missawi, O.; Jeddou, I.B.; Venditti, M.; Zitouni, N.; Zaouali, M.A.; Abdennebi, H.B.; Messaoudi, I.; Reiter, R.J.; Minucci, S.; Banni, M. Environmental microplastic accumulation exacerbates liver ischemia-reperfusion injury in rat: Protective effects of melatonin. Sci. Total Environ. 2023, 860, 160155. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Caradonna, F.; Cruciata, I.; Lauria, A.; Perrone, A.; Gentile, C. Melatonin reduces inflammatory response in hu-man intestinal epithelial cells stimulated by interleukin-1β. J. Pineal Res. 2019, 67, e12598. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.W.; Al-Sadi, R.; Ma, T.Y. IL-1β and the Intestinal Epithelial Tight Junction Barrier. Front. Immunol. 2021, 12, 767456. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Nighot, M.; Al-Sadi, R.; Gupta, Y.; Viszwapriya, D.; Yochum, G.; Koltun, W.; Ma, T.Y. IL1B Increases Intestinal Tight Junction Permeability by Up-regulation of MIR200C-3p, Which Degrades Occludin mRNA. Gastroenterology 2020, 159, 1375–1389. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Ye, D.; Said, H.M.; Ma, T.Y. IL-1β-Induced Increase in Intestinal Epithelial Tight Junction Permeability Is Mediated by MEKK-1 Activation of Canonical NF-κB Pathway. Am. J. Pathol. 2010, 177, 2310–2322. [Google Scholar] [CrossRef]
- Kimura, K.; Teranishi, S.; Nishida, T. Interleukin-1β–Induced Disruption of Barrier Function in Cultured Human Corneal Epi-thelial Cells. Investig. Opthalmol. Vis. Sci. 2009, 50, 597. [Google Scholar] [CrossRef]
- Maria-Ferreira, D.; Nascimento, A.M.; Cipriani, T.R.; Santana-Filho, A.P.; Watanabe, P.D.S.; Sant’ Ana, D.M.G.; Luciano, F.B.; Bocate, K.C.P.; van den Wijngaard, R.M.; Werner, M.F.P.; et al. Rhamnogalacturonan, a chemically-defined polysaccharide, improves intestinal barrier function in DSS-induced colitis in mice and human Caco-2 cells. Sci. Rep. 2018, 8, 12261. [Google Scholar] [CrossRef]
- Cao, J.; Xu, R.; Wang, F.; Geng, Y.; Xu, T.; Zhu, M.; Lv, H.; Xu, S.; Guo, M.Y. Polyethylene microplastics trigger cell apoptosis and inflammation via inducing oxidative stress and activation of the NLRP3 inflammasome in carp gills. Fish Shellfish Immunol. 2023, 132, 108470. [Google Scholar] [CrossRef]
- Yang, W.; Jannatun, N.; Zeng, Y.; Liu, T.; Zhang, G.; Chen, C.; Li, Y. Impacts of microplastics on immunity. Front. Toxicol. 2022, 4, 956885. [Google Scholar] [CrossRef]
- Wang, P.; Qian, H.; Xiao, M.; Lv, J. Role of signal transduction pathways in IL-1β-induced apoptosis: Pathological and therapeutic aspects. Immun. Inflamm. Dis. 2023, 11, e762. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, L.Y.; Chen, Y.; Bai, Y.P.; Jia, J.; Feng, J.G.; Liu, K.X.; Zhou, J. Melatonin alleviates intestinal injury, neuroinflammation and cognitive dysfunction caused by intestinal ischemia/reperfusion. Int. Immunopharmacol. 2020, 85, 106596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Du, L.; Xu, C.; Liu, Q.; Fan, S. The Effects of Melatonin Administration on Intestinal Injury Caused by Abdominal Irradiation from Mice. Int. J. Mol. Sci. 2021, 22, 9715. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [CrossRef]
- Pan, X.; Zhu, L.; Lu, H.; Wang, D.; Lu, Q.; Yan, H. Melatonin Attenuates Oxidative Damage Induced by Acrylamide In Vitro and In Vivo. Oxid. Med. Cell Longev. 2015, 2015, 703709. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Soliman, H.A.; Osman, A.G.; Sayed, A.E. Assessment the effect of exposure to microplastics in Nile Tilapia (Oreo-chromis niloticus) early juvenile: I. blood biomarkers. Chemosphere 2019, 228, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Wilfinger, W.W.; Mackey, K.; Chomczynski, P. Effect of pH and Ionic Strength on the Spectrophotometric Assessment of Nu-cleic Acid Purity. Biotechniques 1997, 22, 474–481. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Khedr, L.H.; Eladawy, R.M.; Nassar, N.N.; Saad, M.A.E. Canagliflozin attenuates chronic unpredictable mild stress induced neuroinflammation via modulating AMPK/mTOR autophagic signaling. Neuropharmacology 2023, 223, 109293. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. The hematoxylin and eosin, connective and mesenchymal tissues with their stains. In Bancroft’s Theory and Practice of Histological Technique, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 126–175. [Google Scholar]
- Sanderson, T.; Wild, G.; Cull, A.M.; Marston, J.; Zardin, G. Immunohistochemical and immunofluoresent techniques. In Ban-croft’s Theory and Practice of Histological Technique, 8th ed.; Suvarna, K.S., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 337–396. [Google Scholar]
- Ayub, B.; Wani, H.; Shoukat, S.; Para, P.; Ganguly, S.; Ali, M. Specimen preparation for electron microscopy: An overview. J. Environ. Life Sci. 2017, 2, 85–88. [Google Scholar]









| Gene Name | Primer Sequences (5’→3’) (F: Forward; R: Reverse) | Accession Number |
|---|---|---|
| Occludin | F: ccttttgagagtccacct R: gtcttccgggtaaaaaga | AB016425.1 |
| MLCK | F: gcacagaaatgggcaaac R: gcttcacaggtgtacttg | NM_001105874.2 |
| ZO-1 | F: tctgatcattccacacag R: tccactgctttgggtgta | NM_001106266 |
| MUC2 | F: acctggggtgacttccact R: atcaggacggactctatg | U68172.1 |
| B-actin | F: ctacctcatgaagatcctcacc R: agttgaaggtagtttcgtggat | NM_007393.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Gazzar, W.B.; Sliem, R.E.; Bayoumi, H.; Nasr, H.E.; Shabanah, M.; Elalfy, A.; Radwaan, S.E.; Gebba, M.A.; Mansour, H.M.; Badr, A.M.; et al. Melatonin Alleviates Intestinal Barrier Damaging Effects Induced by Polyethylene Microplastics in Albino Rats. Int. J. Mol. Sci. 2023, 24, 13619. https://doi.org/10.3390/ijms241713619
El Gazzar WB, Sliem RE, Bayoumi H, Nasr HE, Shabanah M, Elalfy A, Radwaan SE, Gebba MA, Mansour HM, Badr AM, et al. Melatonin Alleviates Intestinal Barrier Damaging Effects Induced by Polyethylene Microplastics in Albino Rats. International Journal of Molecular Sciences. 2023; 24(17):13619. https://doi.org/10.3390/ijms241713619
Chicago/Turabian StyleEl Gazzar, Walaa Bayoumie, Rania E. Sliem, Heba Bayoumi, Hend Elsayed Nasr, Manar Shabanah, Amira Elalfy, Shaimaa E. Radwaan, Mohammed A. Gebba, Heba M. Mansour, Amul M. Badr, and et al. 2023. "Melatonin Alleviates Intestinal Barrier Damaging Effects Induced by Polyethylene Microplastics in Albino Rats" International Journal of Molecular Sciences 24, no. 17: 13619. https://doi.org/10.3390/ijms241713619
APA StyleEl Gazzar, W. B., Sliem, R. E., Bayoumi, H., Nasr, H. E., Shabanah, M., Elalfy, A., Radwaan, S. E., Gebba, M. A., Mansour, H. M., Badr, A. M., Amer, M. F., Ashour, S. S., Morsi, H., Aboelkomsan, E. S. A. F., Baioumy, B., Sayed, A. E. -D. H., & Farag, A. A. (2023). Melatonin Alleviates Intestinal Barrier Damaging Effects Induced by Polyethylene Microplastics in Albino Rats. International Journal of Molecular Sciences, 24(17), 13619. https://doi.org/10.3390/ijms241713619