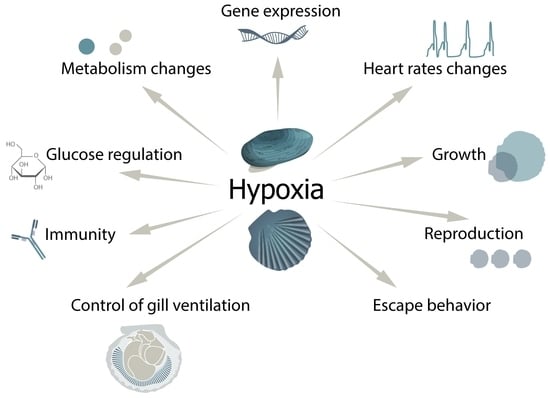
Role of the Neuroendocrine System of Marine Bivalves in Their Response to Hypoxia
Abstract
:1. Introduction
2. Biogenic Amines
2.1. Catecholamines
2.2. Serotonin
3. Acetylcholine
4. Nitric Oxide
5. Hypoxia Inducible Factor-1α
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wijsman, J.W.M.; Troost, K.; Fang, J.; Roncarati, A. Global production of marine bivalves. Trends and challenges. In Goods and Services of Marine Bivalves; Smaal, A., Ferreira, J., Grant, J., Petersen, J., Strand, Ø., Eds.; Springer: Cham, Switzerland, 2019; pp. 7–26. [Google Scholar]
- De Zwaan, A.; Cortesi, P.; Thillart, G.V.D.; Roos, J.; Storey, K. Differential sensitivities to hypoxia by two anoxia-tolerant marine molluscs: A biochemical analysis. Mar. Biol. 1991, 111, 343–351. [Google Scholar] [CrossRef]
- Sobral, P.; Widdows, J. Influence of hypoxia and anoxia on the physiological responses of the clam Ruditapes decussatus from southern Portugal. Mar. Biol. 1997, 127, 455–461. [Google Scholar] [CrossRef]
- Toba, M. The decline of Manila clam stock in Tokyo Bay. Bull. Fish. Res. Agency 2005, 1, 13–18. [Google Scholar]
- Vaquer-Sunyer, R.; Duarte, C.M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA 2008, 105, 15452–15457. [Google Scholar] [CrossRef] [Green Version]
- Sussarellu, R.; Dudognon, T.; Fabioux, C.; Soudant, P.; Moraga, D.; Kraffe, E. Rapid mitochondrial adjustments in response to short-term hypoxia and re-oxygenation in the Pacific oyster Crassostrea gigas. J. Exp. Biol. 2013, 216, 1561–1569. [Google Scholar] [CrossRef] [Green Version]
- Hyvärinen, H.S.; Sjönberg, T.; Marjomäki, T.J.; Taskinen, J. Effect of low dissolved oxygen on the viability of juvenile Margaritifera margaritifera: Hypoxia tolerance ex situ. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1393–1400. [Google Scholar] [CrossRef]
- Babarro, J.M.; De Zwaan, A. Anaerobic survival potential of four bivalves from different habitats. A comparative survey. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Spicer, I.J. What can an ecophysiological approach tell us about the physiological responses of marine invertebrates to hypoxia? J. Exp. Biol. 2014, 217, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Chrousos, G.P. Stressors, stress, and neuroen-docrine integration of the adaptive response. Ann. N.Y. Acad. Sci. 1997, 851, 311–335. [Google Scholar] [CrossRef]
- Wu, R.S. Hypoxia: From molecular responses to ecosystem responses. Mar. Pollut. Bull. 2002, 45, 35–45. [Google Scholar] [CrossRef]
- Larade, K.; Storey, K.B. Chapter 3. A profile of the metabolic responses to anoxia in marine invertebrates. In Cell and Molecular Response to Stress; Elsevier: Amsterdam, The Netherlands, 2002; Volume 3, pp. 27–46. [Google Scholar]
- Kozuki, Y.; Yamanaka, R.; Matsushige, M.; Saitoh, A.; Otani, S.; Ishida, T. The after-effects of hypoxia exposure on the clam Ruditapes philippinarum in Omaehama beach, Japan. Estuar. Coast. Shelf Sci. 2013, 116, 50–56. [Google Scholar] [CrossRef]
- Nie, H.; Wang, H.; Jiang, K.; Yan, X. Transcriptome analysis reveals differential immune related genes expression in Ruditapes philippinarum under hypoxia stress: Potential HIF and NF-κB crosstalk in immune responses in clam. BMC Genom. 2020, 21, 318. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Huang, W.; Luo, Z.; Zheng, C.; Wang, G.; Luo, P.; Wang, S.; Liu, J.; Li, H.; Shu, H. Unraveling the characterization of minichromosome maintenance complex component 2 (MCM2) gene and its SNPs associated with cold-tolerance trait in Pacific white shrimp (Litopenaeus vannamei). Aquac. Rep. 2021, 19, 100610. [Google Scholar] [CrossRef]
- Grieshaber, M.K.; Hardewig, I.; Kreutzer, U.; Pörtner, H.O. Physiological and metabolic responses to hypoxia in invertebrates. Rev. Physiol. Biochem. Pharmacol. 1994, 125, 43–147. [Google Scholar] [PubMed] [Green Version]
- Diaz, R.J.; Rosenberg, R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Ann. Rev. 1995, 33, 245–303. [Google Scholar]
- Wannamaker, C.M.; Rice, J.A. Effects of hypoxia on movements and behavior of selected estuarine organisms from the southeastern United States. J. Exp. Mar. Biol. Ecol. 2000, 249, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Loudon, C. Development of Tenebrio molitor in low oxygen levels. J. Insect Physiol. 1988, 34, 97–103. [Google Scholar] [CrossRef]
- Hochachka, P.; Rupert, J.; Monge, C. Adaptation and conservation of physiological systems in the evolution of human hypoxia tolerance. Comp. Biochem. Physiol. A 1999, 124, 1–17. [Google Scholar] [CrossRef]
- Boutilier, R.G. Mechanisms of cell survival in hypoxia and hypothermia. J. Exp. Biol. 2001, 204, 3171–3181. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Buck, L.T.; Doll, C.J.; Land, S.C. Unifying theory of hypoxia tolerance: Molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl. Acad. Sci. USA 1996, 93, 9493–9498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonanez-Organis, J.G.; Peregrino-Uriarte, A.B.; Gómez-Jiménez, S.; López-Zavala, A.; Forman, H.J.; Yepiz-Plascencia, G. Molecular characterization of hypoxia inducible factor-1 (HIF-1) from the white shrimp Litopenaeus vannamei and tissue-specific expression under hypoxia. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 395–405. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Nesmelova, I.; Larry, L.; Sokolov, E.P.; Sokolova, I.M. Intermittent hypoxia leads to functional reorganization of mitochondria and affects cellular bioenergetics in marine mollusks. J. Exp. Biol. 2016, 219, 1659–1674. [Google Scholar] [CrossRef]
- Willmer, P.; Stone, G.; Johnston, J. Environmental Physiology of Animals; Blackwell Science: Malden, MA, USA, 2000. [Google Scholar]
- McMahon, B.R. Physiological Responses to Oxygen Depletion in Intertidal Animals. Am. Zool. 1988, 28, 39–53. [Google Scholar] [CrossRef]
- Artigaud, S.; Thorne, M.A.; Richard, J.; Lavaud, R.; Jean, F.; Flye-Sainte-Marie, J.; Peck, L.S.; Pichereau, V.; Clark, M.S. Deep sequencing of the mantle transcriptome of the great scallop Pecten maximus. Mar. Genom. 2014, 15, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Hochachka, P.W. Metabolic suppression and oxygen availability. Can. J. Zool. 1988, 66, 152–158. [Google Scholar] [CrossRef]
- Gorr, T.A.; Gassmann, M.; Wappner, P. Sensing and responding to hypoxia via HIF in model invertebrates. J. Insect Physiol. 2006, 52, 349–364. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Yi, Q.; Wang, L.; Song, L. The Neuroendocrine-Immune Regulation in Response to Environmental Stress in Marine Bivalves. Front. Physiol. 2018, 9, 1456. [Google Scholar] [CrossRef] [Green Version]
- Ottaviani, E.; Franceschi, C. The neuroendocrinology of stress from invertebrates to man. Prog. Neurobiol. 1996, 48, 421–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Z.; Wang, L.; Dong, W.; Qiu, L.; Song, L. The cholinergic immune regulation mediated by a novel muscarinic acetylcholine receptor through TNF pathway in oyster Crassostrea gigas. Dev. Comp. Immunol. 2016, 65, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Wang, L.; Gao, Y.; Wang, M.; Zhang, H.; Wang, L.; Qiu, L.; Song, L. A monoamine oxidase from scallop Chlamys farreri serving as an immunomodulator in response against bacterial challenge. Dev. Comp. Immunol. 2011, 35, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, L.; Shi, X.; Zhang, H.; Gao, Y.; Wang, M.; Kong, P.; Qiu, L.; Song, L. The modulation of catecholamines to the immune response against bacteria Vibrio anguillarum challenge in scallop Chlamys farreri. Fish Shellfish Immunol. 2011, 31, 1065–1071. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Shi, X.; Yue, F.; Wang, M.; Zhang, H.; Song, L. The expression of dopa decarboxylase and dopamine beta hydroxylase and their responding to bacterial challenge during the ontogenesis of scallop Chlamys farreri. Fish Shellfish Immunol. 2012, 33, 67–74. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, Z.; Wang, L.; Yue, F.; Wang, M.; Yang, C.; Song, L. The Immunomodulation of Acetylcholinesterase in Zhikong Scallop Chlamys farreri. PLoS ONE 2012, 7, e30828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Wang, L.; Zhou, Z.; Wang, M.; Liu, R.; Wang, L.; Jiang, Q.; Song, L. An opioid growth factor receptor (OGFR) for [Met5]-enkephalin in Chlamys farreri. Fish Shellfish Immunol. 2013, 34, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, Z.; Wang, L.; Wang, M.; Shi, S.; Wang, Z.; Song, L. The immunomodulation of nicotinic acetylcholine receptor subunits in Zhikong scallop Chlamys farreri. Fish Shellfish Immunol. 2015, 47, 611–622. [Google Scholar] [CrossRef]
- Rajashekhar, K.P.; Wilkens, J.L. Dopamine and nicotine, but not serotonin, modulate the crustacean ventilatory pattern generator. J. Neurobiol. 1992, 23, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, M.S.; Wilkens, J.L. The role of the cardioregulatory nerves in mediating heart-rate responses to locomotion, reduced stroke volume, and neurohormones in Homarus americanus. Biol. Bull. 1995, 188, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, J.; Kuramoto, T.; McMahon, B. The effects of six pericardial hormones and hypoxia on the semi-isolated heart and sternal arterial valve of the lobster Homarus americanus. Comp. Biochem. Physiol. C 1996, 114, 57–65. [Google Scholar] [CrossRef]
- Kuo, C.M.; Yang, Y.H. Hyperglycemic responses to cold shock in the freshwater giant prawn, Macrobrachium rosenbergii. J. Comp. Physiol. B 1999, 169, 49–54. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Gold, P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. J. Am. Med. Assoc. 1992, 267, 1244–1252. [Google Scholar] [CrossRef]
- Bonga, S.E.W. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Giovine, M.; Pozzolini, M.; Favre, A.; Bavestrello, G.; Cerrano, C.; Ottaviani, F.; Chiarantini, L.; Cerasi, A.; Cangiotti, M.; Zocchi, E.; et al. Heat Stress-Activated, Calcium-Dependent Nitric Oxide Synthase in Sponges. Nitric Oxide Biol. Chem. 2001, 5, 427–431. [Google Scholar] [CrossRef] [PubMed]
- González, P.M.; Rocchetta, I.; Abele, D.; Rivera-Ingraham, G.A. Hypoxically Induced Nitric Oxide: Potential Role as a Vasodilator in Mytilus edulis Gills. Front. Physiol. 2019, 9, 1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Guo, R.; Powell-Coffman, J.A. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc. Natl. Acad. Sci. USA 2001, 98, 7916–7921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, K.M.; Follett, C.R.; Burnett, L.E.; Lema, S.C. Gene transcripts encoding hypoxia-inducible factor (HIF) exhibit tissueand muscle fiber type-dependent responses to hypoxia and hypercapnic hypoxia in the Atlantic blue crab, Callinectes sapidus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 163, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Kodama, K.; Rahman, M.S.; Horiguchi, T.; Thomas, P. Assessment of hypoxia-inducible factor-1α mRNA expression in mantis shrimp as a biomarker of environmental hypoxia exposure. Biol. Lett. 2012, 8, 278–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannetto, A.; Maisano, M.; Cappello, T.; Oliva, S.; Parrino, V.; Natalotto, A.; De Marco, G.; Barberi, C.; Romeo, O.; Mauceri, A.; et al. Hypoxia-Inducible Factor α and Hif-prolyl Hydroxylase Characterization and Gene Expression in Short-Time Air-Exposed Mytilus galloprovincialis. Mar. Biotechnol. 2015, 17, 768–781. [Google Scholar] [CrossRef]
- Sinakevitch, I.T.; Wolff, G.H.; Pflüger, H.-J.; Smith, B.H. Editorial: Biogenic Amines and Neuromodulation of Animal Behavior. Front. Syst. Neurosci. 2018, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Budelmann, B.-U.; Bonn, U. Histochemical evidence for catecholamines as neurotransmitters in the statocyst of Octopus vulgaris. Cell Tissue Res. 1982, 227, 475–483. [Google Scholar] [CrossRef]
- Williamson, R. Electrophysiological evidence for cholinergic and catecholaminergic efferent transmitters in the statocyst of octopus. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1989, 93, 23–27. [Google Scholar] [CrossRef]
- Kvetnansky, R.; Sabban, E.L.; Palkovits, M. Catecholaminergic Systems in Stress: Structural and Molecular Genetic Approaches. Physiol. Rev. 2009, 89, 535–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, H.; Xu, B.; Wang, F.; Liu, B. Catecholaminergic responses to environmental stress in the hemolymph of zhikong scallop Chlamys farreri. J. Exp. Zool. A Ecol. Genet. Physiol. 2008, 309, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, Z.; Qiu, L.; Wang, W.; Song, X.; Wang, X.; Li, Y.; Xin, L.; Wang, L.; Song, L. The modulation role of serotonin in Pacific oyster Crassostrea gigas in response to air exposure. Fish Shellfish Immunol. 2017, 62, 341–348. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, E.F.; Watson, W.H.; Wyse, G.A. Identification and localization of catecholamines in the nervous system of Limulus polyphemus. J. Neurobiol. 1982, 13, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Franchini, A.; Ottaviani, E.; Caselgrandi, E. Biogenic amines in the snail brain of Helicella virgata (Gastropoda, Pulmonata). Brain Res. 1985, 347, 132–134. [Google Scholar] [CrossRef]
- Pani, A.K.; Croll, R. Distribution of catecholamines, indoleamines, and their precursors and metabolites in the scallop, Placopecten magellanicus (Bivalvia, Pectinidae). Cell. Mol. Neurobiol. 1995, 15, 371–386. [Google Scholar] [CrossRef]
- Kniazkina, M.; Dyachuk, V. Neurogenesis of the scallop Azumapecten farreri: From the frst larval sensory neurons to the defnitive nervous system of juveniles. Front. Zool. 2022, 19, 22. [Google Scholar] [CrossRef]
- Lacoste, A.; Malham, S.; Cueff, A.; Jalabert, F.; Gelebart, F.; Poulet, S. Evidence for a form of adrenergic response to stress in the mollusc Crassostrea gigas. J. Exp. Biol. 2001, 204, 1247–1255. [Google Scholar] [CrossRef]
- Stefano, G.B.; Aiello, E. Histofluorescent localization of serotonin and dopamine in the nervous system and gill of Mytilus edulis (Bivalvia). Biol. Bull. 1975, 148, 141–156. [Google Scholar] [CrossRef]
- Smith, S.A.; Nason, J.; Croll, R.P. Distribution of catecholamines in the sea scallop, Placopecten magellanicus. Can. J. Zool. 1998, 76, 1254–1262. [Google Scholar] [CrossRef]
- Matsutani, T.; Nomura, T. Localization of monoamines in the central nervous system and gonad of the scallop Patinopecten yessoensis. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 425–430. [Google Scholar] [CrossRef]
- Kotsyuba, E.P. Effects of temperature stress on NO-synthase and tyrosine hydroxylase activities in the central nervous system of bivalve molluscs. J. Evol. Biochem. Physiol. 2009, 45, 138–146. [Google Scholar] [CrossRef]
- Stefano, G.B.; Hiripi, L.; Catapane, E.J. The effects of short and long term temperature stress on serotonin, dopamine and norepinephrine metabolism in molluscan ganglia. J. Therm. Biol. 1978, 3, 79–83. [Google Scholar] [CrossRef]
- Lacoste, A.; Malham, S.K.; Cueff, A.; Poulet, S.A. Stress-Induced Catecholamine Changes in the Hemolymph of the Oyster Crassostrea gigas. Gen. Comp. Endocrinol. 2001, 122, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Lansing, M.B.; Gardner, W.S.; Eadie, B.J. Catecholamines as potential sub-lethal stress indicators in Great Lakes macrobenthic invertebrates. J. Great Lakes Res. 1993, 19, 569–587. [Google Scholar] [CrossRef]
- Maule, A.G.; Vanderkooi, S.P. Stress-induced immune-endocrine interaction. In Stress Physiology in Animals; Balm, P., Ed.; Sheffield Academic Press Ltd.: Sheffield, UK, 1999; pp. 205–245. [Google Scholar]
- Malham, S.K.; Lacoste, A.; Gélébart, F.; Cueff, A.; Poulet, S.A. A first insight into stress-induced neuroendocrine and immune changes in the octopus Eledone cirrhosa. Aquat. Living Resour. 2002, 15, 187–192. [Google Scholar] [CrossRef]
- Kinkead, R.; Fritsche, R.; Perry, S.F.; Nilsson, S. The Role of Circulating Catecholamines in the Ventilatory and Hypertensive Responses to Hypoxia in the Atlantic Cod (Gadus morhua). Physiol. Zool. 1991, 64, 1087–1109. [Google Scholar] [CrossRef]
- Carroll, M.A.; Catapane, E.J. The nervous system control of lateral ciliary activity of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Bayne, B. Biology of Oysters (Developments in Aquaculture and Fisheries Science), 1st ed.; Academic Press: London, UK, 2017. [Google Scholar]
- Catapane, E.J.; Stefano, G.B.; Aiello, E. Pharmacological Study of the Reciprocal Dual Innervation of the Lateral Ciliated Gill Epithelium by the Cns of Mytilus edulis (Bivalvia). J. Exp. Biol. 1978, 74, 101–113. [Google Scholar] [CrossRef]
- Aiello, E.L. Nervous control of gill ciliary activity in Mytilus edulis. In Neurobiology of Mytilus edulis; Stefano, G.B., Ed.; Manchester University Press: Manchester, UK, 1990; Volume 10, pp. 189–208. [Google Scholar]
- Marinković, M.; Berger, J.; Jékely, G. Neuronal coordination of motile cilia in locomotion and feeding. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190165. [Google Scholar] [CrossRef] [Green Version]
- Martin, K.; Huggins, T.; King, C.; Carroll, M.A.; Catapane, E.J. The neurotoxic effects of manganese on the dopaminergic innervation of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. Part C 2008, 148, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, M.; Huggins, T.; Licorish, R.; Carroll, M.A.; Catapane, E.J. Effects of p-Aminosalicylic acid on the neurotoxicity of manganese on the dopaminergic innervation of the cilia of the lateral cells of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. Part C 2010, 151, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.I.; Rich, D.R.; Muruganathan, S.P.; Liu, M.B.; Pon, J.R.; Tam, R.; Diefenbach, T.J.; Kuang, S. Identification and evolutionary implications of neurotransmitter-interactions underlying the behavioral response to hypoxia in Lymnaea stagnalis embryos. J. Exp. Biol. 2011, 214, 2660–2670. [Google Scholar] [CrossRef] [Green Version]
- Soliman, S. Pharmacological control of ciliary activity in the young sea urchin larva. Effects of monoaminergic agents. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1983, 76, 181–191. [Google Scholar] [CrossRef]
- Katow, H.; Suyemitsu, T.; Ooka, S.; Yaguchi, J.; Jin-Nai, T.; Kuwahara, I.; Katow, T.; Yaguchi, S.; Abe, H. Development of a dopaminergic system in sea urchin embryos and larvae. J. Exp. Biol. 2010, 213, 2808–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, E.A.; Bainy, A.C.; Medeiros, M.H.; Di Mascio, P. Effects of trace metal and exposure to air on serotonin and dopamine levels in tissues of the mussel Perna perna. Mar. Pollut. Bull. 2003, 46, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Nemcsók, J.; Hiripi, L.; Patocskai, M.; Salánki, J.; Kufcsák, O. The effects of pesticides on monoaminergic system related to periodic activity of mussels (Anodonta cygnea L.). Gen. Pharmacol. 1997, 29, 79–83. [Google Scholar] [CrossRef]
- Sang, T.-K.; Chang, H.-Y.; Lawless, G.M.; Ratnaparkhi, A.; Mee, L.; Ackerson, L.C.; Maidment, N.T.; Krantz, D.E.; Jackson, G.R. A Drosophila Model of Mutant Human Parkin-Induced Toxicity Demonstrates Selective Loss of Dopaminergic Neurons and Dependence on Cellular Dopamine. J. Neurosci. 2007, 27, 981–992. [Google Scholar] [CrossRef] [Green Version]
- Coulom, H.; Birman, S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 2004, 24, 10993–10998. [Google Scholar] [CrossRef] [Green Version]
- Vehovszky, A.; Szabo, H.; Hiripi, L.; Elliott, C.J.H.; Hernadi, L. Behavioural and neural deficits induced by rotenone in the pond snail Lymnaea stagnalis. A possible model for Parkinson’s disease in an invertebrate. Eur. J. Neurosci. 2007, 25, 2123–2130. [Google Scholar] [CrossRef]
- Wu, W.-H.; Cooper, R.L. Serotonin and Synaptic Transmission at Invertebrate Neuromuscular Junctions. Exp. Neurobiol. 2012, 21, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Voigt, J.-P.; Fink, H. Serotonin controlling feeding and satiety. Behav. Brain Res. 2015, 277, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.J. Feeding, hunger, satiety and serotonin in invertebrates. Proc. R. Soc. B 2020, 287, 20201386. [Google Scholar] [CrossRef]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.-P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, D.W.; Pinsker, H.M. Swimming in Aplysia brasiliana: Behavioral and cellular efects of serotonin. J. Neurophysiol. 1989, 62, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, J.M.; Katz, P.S. Diferent functions for homologous serotonergic interneurons and serotonin in species-specific rhythmic behaviours. Proc. Biol. Sci. 2009, 276, 99–108. [Google Scholar]
- Lewis, S.L.; Lyons, D.E.; Meekins, T.L.; Newcomb, J.M. Serotonin Influences Locomotion in the Nudibranch Mollusc Melibe leonina. Biol. Bull. 2011, 220, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alavi, S.M.H.; Nagasawa, K.; Takahashi, K.G.; Osada, M. Structure-function of serotonin in bivalve molluscs. In Serotonin—A Chemical Messenger between All Types of Living Cells; InTech: London, UK, 2017. [Google Scholar]
- Il-Han, J.; Janes, T.; Lukowiak, K. The role of serotonin in the enhancement of long-term memory resulting from predator detection in Lymnaea. J. Exp. Biol. 2010, 213, 3603–3614. [Google Scholar] [CrossRef] [Green Version]
- Matsutani, T.; Nomura, T. Serotonin-like immunoreactivity in the central nervous system and gonad of the scallop, Patinopecten yessoensis. Cell Tissue Res. 1986, 244, 515–517. [Google Scholar] [CrossRef]
- Vitellaro-Zuccarello, L.; De Biasi, S.; Bernardi, P.; Oggioní, A. Distribution of serotonin-, gamma-aminobutyric acid- and substance P-like immunoreactivity in the central and peripheral nervous system of Mytilus galloprovincialis. Tissue Cell 1991, 23, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Croll, R.P.; Too, C.K.; Pani, A.K.; Nason, J. Distribution of serotonin in the sea scallop Placopecten magellanicus. Invertebr. Reprod. Dev. 1995, 28, 125–135. [Google Scholar] [CrossRef]
- Siniscalchi, A.; Cavallini, S.; Sonetti, D.; Sbrenna, G.; Capuano, S.; Barbin, L.; Turolla, E.; Rossi, R. Serotonergic neurotransmission in the bivalve Venus verrucosa (Veneridae): A neurochemical and immunohistochemical study of the visceral ganglion and gonads. Mar. Biol. 2004, 144, 1205–1212. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, B.; Dong, W.; Liu, Z.; Lv, Z.; Jia, Z.; Qiu, L.; Wang, L.; Song, L. A serotonin receptor (Cg5-HTR-1) mediating immune response in oyster Crassostrea gigas. Dev. Comp. Immunol. 2018, 82, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kotsyuba, E.; Kalachev, A.; Kameneva, P.; Dyachuk, V. Distribution of Molecules Related to Neurotransmission in the Nervous System of the Mussel Crenomytilus grayanus. Front. Neuroanat. 2020, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Catapane, E.J. Seasonal monoamine changes in the central nervous system of Mytilus edulis (Bivalvia). Experientia 1977, 33, 1341–1342. [Google Scholar] [CrossRef]
- Klouche, M.S.; De Deurwaerdère, P.; Dellu-Hagedorn, F.; Lakhdar-Ghazal, N.; Benomar, S. Monoamine content during the reproductive cycle of Perna perna depends on site of origin on the Atlantic Coast of Morocco. Sci. Rep. 2015, 5, 13715. [Google Scholar] [CrossRef] [Green Version]
- Kotsyuba, E.; Dyachuk, V. Effect of Air Exposure-Induced Hypoxia on Neurotransmitters and Neurotransmission Enzymes in Ganglia of the Scallop Azumapecten farreri. Int. J. Mol. Sci. 2022, 23, 2027. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.E.; Prior, G. Dynein from serotonin-activated cilia and flagella: Extraction characteristics and distinct sites for cAMP-dependent protein phosphorylation. J. Cell Sci. 1992, 103, 999–1012. [Google Scholar] [CrossRef]
- Gainey, L.F.; Greenberg, M.J. Nitric oxide mediates seasonal muscle potentiation in clam gills. J. Exp. Biol. 2003, 206, 3507–3520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay-Schmidt, A. The evolution of the serotonergic nervous system. Proc. R. Soc. B 2000, 267, 1071–1079. [Google Scholar] [CrossRef]
- Kuang, S.; Doran, S.A.; Wilson, R.J.A.; Goss, G.G.; Goldberg, J.I. Serotonergic sensory-motor neurons mediate a behavioral response to hypoxia in pond snail embryos. J. Neurobiol. 2002, 52, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Koss, R.; Diefenbach, T.J.; Kuang, S.; Doran, S.A.; Goldberg, J.I. Coordinated development of identified serotonergic neurons and their target ciliary cells in Helisoma trivolvis embryos. J. Comp. Neurol. 2003, 457, 313–325. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rundle, S.D.; Smirthwaite, J.J.; Spicer, J.I. Embryonic rotational behaviour in the pond snail Lymnaea stagnalis: Influences of environmental oxygen and development stage. Zoology 2009, 112, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.I.; Doran, S.A.; Shartau, R.B.; Pon, J.R.; Ali, D.W.; Tam, R.; Kuang, S. Integrative biology of an embryonic respiratory behaviour in pond snails: The ‘embryo stir-bar hypothesis’. J. Exp. Biol. 2008, 211, 1729–1736. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, J.I.; Koehncke, N.K.; Christopher, K.J.; Neumann, C.; Diefenbach, T.J. Pharmacological characterization of a serotonin receptor involved in an early embryonic behavior of Helisoma trivolvis. J. Neurobiol. 1994, 25, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Mapara, S.; Parries, S.; Quarrington, C.; Ahn, K.-C.; Gallin, W.J.; Goldberg, J.I. Identification, molecular structure and expression of two cloned serotonin receptors from the pond snail, Helisoma trivolvis. J. Exp. Biol. 2008, 211, 900–910. [Google Scholar] [CrossRef] [Green Version]
- Berlind, A. Neurohumoral and reflex control of scaphognathite beating in the crabCarcinus maenas. J. Comp. Physiol. A 1977, 116, 77–90. [Google Scholar] [CrossRef]
- Bayne, B. Ventilation, the heart beat and oxygen uptake by Mytilus edulis L. in declining oxygen tension. Comp. Biochem. Physiol. Part A Physiol. 1971, 40, 1065–1085. [Google Scholar] [CrossRef]
- Koester, J.; Dieringer, N.; Mandelbaum, D. Cellular Neuronal Control of Molluscan Heart. Am. Zool. 1979, 19, 103–116. [Google Scholar] [CrossRef] [Green Version]
- Kuwasawa, K.; Hill, R. Evidence for cholinergic inhibitory and serotonergic excitatory neuromuscular transmission in the heart of the bivalve Mercenaria mercenaria. J. Exp. Biol. 1997, 200, 2123–2135. [Google Scholar] [CrossRef]
- Kodirov, S.A. The neuronal control of cardiac functions in Molluscs. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 160, 102–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, E.; Capuzzo, A. Cyclic AMP Signaling in Bivalve Molluscs: An Overview. J. Exp. Zool. A Ecol. Genet. Physiol. 2010, 313, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Sun, C.; Rong, Y.; Wang, H.; Xu, Q.; Gao, F.; Wang, A. Circulatory and Metabolic Physiology Disorder in Different Organs of the Subtropical Scallop Species Chlamys nobilis Under Thermal and Hypoxia Stress, Revealed by Doppler Ultrasonography Technique. Front. Mar. Sci. 2022, 9, 880112. [Google Scholar] [CrossRef]
- Nicholson, S. Ecophysiological aspects of cardiac activity in the subtropical mussel Perna viridis (L.) (Bivalvia: Mytilidae). J. Exp. Mar. Biol. Ecol. 2002, 267, 207–222. [Google Scholar] [CrossRef]
- Michaelidis, B.; Storey, K. Phosphofructokinase from the anterior byssus retractor muscle of Mytilvs edulis: Modification of the enzyme in anoxia and by endogenous protein kinases. Int. J. Biochem. 1990, 22, 759–765. [Google Scholar] [CrossRef]
- Canesi, L.; Miglioli, A.; Balbi, T.; Fabbri, E. Physiological Roles of Serotonin in Bivalves: Possible Interference by Environmental Chemicals Resulting in Neuroendocrine Disruption. Front. Endocrinol. 2022, 13, 792589. [Google Scholar] [CrossRef]
- Odintsova, N.; Dyachuk, V.; Kiselev, K.; Sheludko, N. Expression of thick filament proteins during ontogenesis of the mussel Mytilus trossulus (Mollusca: Bivalvia). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 144, 238–244. [Google Scholar] [CrossRef]
- Dyachuk, V.; Wanninger, A.; Voronezhskaya, E.E. Innervation of bivalve larval catch muscles by serotonergic and FMRFamidergic neurons. Acta Biol. Hung. 2012, 63, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, S.S.; Shevchenko, U.V.; Dyachuk, V.A.; Vyatchin, I.G. A Preparative Method for the Isolation of Calponin from Molluscan Catch Muscle. Int. J. Mol. Sci. 2022, 23, 7993. [Google Scholar] [CrossRef]
- Twarog, B.M. Responses of a molluscan smooth muscle to acetylcholine and 5-hydroxytriptamine. J. Cell Physiol. 1954, 44, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, G.; Lowy, J. The Paradox of Mytilus Muscle: A New Interpretation. J. Exp. Biol. 1956, 33, 295–310. [Google Scholar] [CrossRef]
- York, B.; Twarog, B.M. Evidence for the release of serotonin by relaxing nerves in molluscan muscle. Comp. Biochem. Physiol. Part A Physiol. 1973, 44, 423–430. [Google Scholar] [CrossRef]
- Achazi, R.K.; Dolling, B.; Haakshorst, R. 5HT-induced relaxation and cyclic AMP in a molluscan smooth muscle. Pflug. Arch. Eur. J. Phys. 1974, 349, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Siegman, M.J.; Mooers, S.T.J.; Li, C.; Narayan, S.; Trincle-Mulcahy, L.; Watabe, S.; Hartshorne, D.J.; Butler, T.M. Phosphorylation of a high molecular weight (approximately 600 kDa) protein regulates catch in invertebrate smooth muscle. J. Muscle Res. Cell Motil. 1997, 18, 655–670. [Google Scholar] [CrossRef]
- D’Este, L.; Kimura, S.; Casini, A.; Matsuo, A.; Bellier, J.-P.; Kimura, H.; Renda, T.G. First visualization of cholinergic cells and fibers by immunohistochemistry for choline acetyltransferase of the common type in the optic lobe and peduncle complex of Octopus vulgaris. J. Comp. Neurol. 2008, 509, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.K. Hypoxia. 3. Hypoxia and neurotransmitter synthesis. Am. J. Physiol. Cell. Physiol. 2011, 300, C743–C751. [Google Scholar] [CrossRef] [Green Version]
- Halder, N.; Lal, G. Cholinergic System and Its Therapeutic Importance in Inflammation and Autoimmunity. Front. Immunol. 2021, 12, 660342. [Google Scholar] [CrossRef]
- Messenger, J.B. Neurotransmitters of cephalopods. Invertebr. Neurosci. 1996, 2, 94–114. [Google Scholar] [CrossRef]
- Bellier, J.P.; Casini, A.; Sakaue, Y.; Kimura, S.; Kimura, H.; Renda, T.G.; D’este, L. Chemical neuroanatomy of the cholinergic neurons in the cephalopod octopus and the gastropod Limax. In Mollusks: Morphology, Behavior and Ecology; Nova Science Publishers: New York, NY, USA, 2012. [Google Scholar]
- Deiana, S.; Platt, B.; Riedel, G. The cholinergic system and spatial learning. Behav. Brain Res. 2011, 221, 389–411. [Google Scholar] [CrossRef]
- van Nierop, P.; Bertrand, S.; Munno, D.W.; Gouwenberg, Y.; Van Minnen, J.; Spafford, J.D.; Syed, N.I.; Bertrand, D.; Smit, A.B. Identification and functional expression of a family of nicotinic acetylcholine receptor subunits in the central nervous system of the mollusk Lymnaea stagnalis. J. Biol. Chem. 2006, 281, 1680–1691. [Google Scholar] [CrossRef] [Green Version]
- Kiss, T.; Krajcs, N.; Pirger, Z.; Hernádi, L. Nicotinic acetylcholine receptors containing the α7-like subunit mediate contractions of muscles responsible for space positioning of the snail, Helix pomatia L. tentacle. PLoS ONE 2014, 9, e109538. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Cao, Y.; Zheng, Z.; Liu, M.; Guo, X. Massive expansion and diversity of nicotinic acetylcholine receptors in lophotrochozoans. BMC Genom. 2019, 20, 937. [Google Scholar] [CrossRef] [Green Version]
- Casini, A.; Vaccaro, R.; D’Este, L.; Sakaue, Y.; Bellier, J.; Kimura, H.; Renda, T. Immunolocalization of choline acetyltransferase of common type in the central brain mass of Octopus vulgaris. Eur. J. Histochem. 2012, 56, 34. [Google Scholar] [CrossRef]
- Sakaue, Y.; Bellier, J.-P.; Kimura, S.; D’Este, L.; Takeuchi, Y.; Kimura, H. Immunohistochemical localization of two types of choline acetyltransferase in neurons and sensory cells of the octopus arm. Brain Struct. Funct. 2014, 219, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Norekian, T.P. Coordination of Startle and Swimming Neural Systems in the Pteropod Mollusk Clione limacina: Role of the Cerebral Cholinergic Interneuron. J. Neurophysiol. 1997, 78, 308–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norekian, T.P.; Satterlie, R.A. Cholinergic Activation of Startle Motoneurons by a Pair of Cerebral Interneurons in the Pteropod Mollusk Clione limacina. J. Neurophysiol. 1997, 77, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhang, F.; Wang, M.; Li, M.; Sun, S. Effects of hypoxia on survival, behavior, and metabolism of Zhikong scallop Chlamys farreri Jones et Preston 1904. J. Oceanol. Limnol. 2019, 38, 351–363. [Google Scholar] [CrossRef]
- Köhler, G.; Lindl, T. Effects of 5-hydroxytryptamine, dopamine, and acetylcholine on accumulation of cyclic AMP and cyclic GMP in the anterior byssus retractor muscle of Mytilus edulis L. (Molluscana). Pflug. Arch. 1980, 383, 257–262. [Google Scholar] [CrossRef]
- Jewell, B.R. The nature of the phasic and the tonic responses of the anterior byssal retractor muscle of Mytilus. J. Physiol. 1959, 149, 154–177. [Google Scholar] [CrossRef]
- Sugi, H.; Ohno, T.; Moriya, M. Mechanism and Function of the Catch State in Molluscan Smooth Muscle: A Historical Perspective. Int. J. Mol. Sci. 2020, 21, 7576. [Google Scholar] [CrossRef] [PubMed]
- De Zwaan, A.; Cortesi, P.; van den Thillart, G.; Brooks, S.; Storey, K.B.; Roos, J.; van Lieshout, G.; Cattani, O.; Vitali, G. Energy metabolism of bivalves at reduced oxygen tensions. In Marine Coastal Eutrophication; Vollenweider, R.A., Marchetti, R., Viviani, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 1029–1039. [Google Scholar]
- Raineri, M. Is a mollusc an evolved bent metatrochophore? A histochemical investigation of neurogenesis in Mytilus (Mollusca: Bivalvia). J. Mar. Biol. Assoc. UK 1995, 75, 571–592. [Google Scholar] [CrossRef]
- Bulbring, E.; Burn, J.H.; Shelly, H.J. Acetylcholine and ciliary movement in the gill plates of Mytilus edulis. Proc. Roy. Soc. Lond. Ser. B 1953, 141, 445–466. [Google Scholar]
- Aiello, E.; Paparo, A. A role for acetylcholine in the regulation of ciliary activity. Cotnp. Gen. Pharsnacol. 1974, 5, 285–297. [Google Scholar] [CrossRef]
- Maisano, M.; Natalotto, A.; Cappello, T.; Giannetto, A.; Oliva, S.; Parrino, V.; Sanfilippo, M.; Mauceri, A. Influences of Environmental Variables on Neurotransmission, Oxidative System, and Hypoxia Signaling on Two Clam Species from a Mediterranean Coastal Lagoon. J. Shellfish Res. 2016, 35, 41–49. [Google Scholar] [CrossRef]
- Beley, A.; Bertrand, N.; Beley, P. Cerebral ischemia: Changes in brain choline, acetylcholine, and other monoamines as related to energy metabolism. Neurochem. Res. 1991, 16, 555–561. [Google Scholar] [CrossRef]
- Jope, R.S.; Jenden, D.J. Choline and phospholipid metabolism and the synthesis of acetylcholine in rat brain. J. Neurosci. Res. 1979, 4, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Blusztajn, J.K.; Liscovitch, M.; Richardson, U.I. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc. Natl. Acad. Sci. USA 1987, 84, 5474–5477. [Google Scholar] [CrossRef] [Green Version]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Wang, R.; Wang, X.; Cong, P.; Liu, Y.; Li, Z.; Xu, J.; Xue, C. Preparation and effects on neuronal nutrition of plasmenylethonoamine and plasmanylcholine from the mussel Mytilus edulis. Biosci. Biotechnol. Biochem. 2020, 84, 380–392. [Google Scholar] [CrossRef]
- Jin, X.; Wang, R.-H.; Wang, H.; Long, C.-L.; Wang, H. Brain protection against ischemic stroke using choline as a new molecular bypass treatment. Acta Pharmacol. Sin. 2015, 36, 1416–1425. [Google Scholar] [CrossRef] [Green Version]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Stefano, G.B.; Ottaviani, E. The biochemical substrate of nitric oxide signaling is present in primitive non-cognitive organisms. Brain Res. 2002, 924, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A. Nitric oxide in marine invertebrates: A comparative perspective. Comp. Biochem. Physiol. Part AMol. Integr. Physiol. 2005, 142, 241–248. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, D.-Y. Neuronal nitric oxide synthase: Structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 2009, 20, 223–230. [Google Scholar] [CrossRef]
- Colasanti, M.; Venturini, G. Nitric oxide in invertebrates. Mol. Neurobiol. 1998, 17, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Ayajiki, K. Phylogenesis of constitutively formed nitric oxide in nonmammals. Rev. Physiol. Biochem. Pharmacol. 2006, 138, 31–80. [Google Scholar]
- Wright, N.J.D. A review of the actions of Nitric Oxide in development and neuronal function in major invertebrate model systems. AIMS Neurosci. 2019, 6, 146–174. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhou, Z.; Wang, L.; Wang, L.; Yue, F.; Wang, J.; Song, L. A Scallop Nitric Oxide Synthase (NOS) with Structure Similar to Neuronal NOS and Its Involvement in the Immune Defense. PLoS ONE 2013, 8, e69158. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Liu, Z.; Zhou, Z.; Wang, L.; Wang, L.; Yue, F.; Wang, J.; Wang, H.; Song, L. Transcriptional activation and translocation of ancient NOS during immune response. FASEB J. 2016, 30, 3527–3540. [Google Scholar] [CrossRef] [Green Version]
- Bredt, D.S.; Glatt, C.E.; Hwang, P.M.; Fotuhi, M.; Dawson, T.M.; Snyder, S.H. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron 1991, 7, 615–624. [Google Scholar] [CrossRef]
- Dawson, T.M.; Bredt, D.S.; Fotuhi, M.; Hwang, P.M.; Snyder, S.H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc. Natl. Acad. Sci. USA 1991, 88, 7797–7801. [Google Scholar] [CrossRef] [Green Version]
- Hope, B.T.; Michael, G.I.; Knigge, K.M.; Vinsent, S.R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1991, 88, 2811–2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroz, L.L.; Gillette, R. From Polyplacophora to Cephalopoda: Comparative analysis of nitric oxide signalling systems in Mollusca. Acta Biol. Hung. 1995, 46, 169–182. [Google Scholar]
- Moroz, L.L.; Chen, D.; Gillette, M.U.; Gillette, R. Nitric Oxide Synthase Activity in the Molluscan CNS. J. Neurochem. 1996, 66, 873–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newcomb, J.M.; Watson, W.H. Modulation of swimming in the gastropod Melibe leonina by nitric oxide. J. Exp. Biol. 2002, 205, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Batista, L.A.; Hoppes, J.L.; Lee, K.J.; Mykles, D.L. A crustacean nitric oxide synthase expressed in nerve ganglia, Y-organ,gill and gonad of the tropical land crab, Gecarcinus lateralis. J. Exp. Biol. 2004, 207, 2845–2857. [Google Scholar] [CrossRef]
- Vaschenko, M.; Kotsyuba, E. NADPH-diaphorase activity in the central nervous system of the Gray mussel Crenomytilus grayanus (Dunker) under stress conditions: A histochemical study. Mar. Environ. Res. 2008, 66, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benton, J.L.; Sandeman, D.C.; Beltz, B.S. Nitric oxide in the crustacean brain: Regulation of neurogenesis and morphogenesis in the developing olfactory pathway developmental dynamics. Develop. Dyn. 2007, 236, 3047–3060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, A.; Ottaviani, E. Nitric oxide synthase activity in molluscan hemocytes. FEBS Lett. 1995, 365, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Jacklet, J.W. Nitric oxide signaling in invertebrates. Invertebr. Neurosci. 1997, 3, 1–14. [Google Scholar] [CrossRef]
- Dyuizen, I.V.; Annikova, L.V.; Motavkin, P.A. NO-synthase localization in the central nervous system of the bivalve mollusks Mizuhopecten yessoensis and Modiolus kurilensis. Russ. J. Mar. Biol. 1999, 25, 277–279. [Google Scholar]
- Tafalla, C.; Gómez-León, J.; Novoa, B.; Figueras, A. Nitric oxide production by carpet shell clam (Ruditapes decussatus) hemocytes. Dev. Comp. Immunol. 2003, 27, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Abele, D.; Puntarulo, S. Iron and radical content in Mya arenaria: Possible sources of NO generation. Aquat. Toxicol. 2008, 89, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strahl, J.; Abele, D. Nitric oxide mediates metabolic functions in the bivalve Arctica islandica under hypoxia. PLoS ONE 2020, 15, e0232360. [Google Scholar] [CrossRef]
- Smith, K.L.; Galloway, T.S.; Depledge, M.H. Neuro-endocrine biomarkers of population-induced stress in marine invertebrates. Sci. Total. Environ. 2000, 262, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Olgart, C.; Gustafsson, L.E.; Wiklund, N.P. Evidence for nonvesicular nitric oxide release evoked by nerve activation. Eur. J. Neurosci. 2000, 12, 1303–1309. [Google Scholar] [CrossRef]
- Lipton, S.A. Neuronal protection and destruction by NO. Cell Death Differ. 1999, 6, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Payne, R.S.; Haine, M.F.; Rigor, B.M. Hypoxia, excitoxicity and neuroprotection in the hippocampal slice preparation. J. Neurosci. Meth. 1995, 59, 129–138. [Google Scholar] [CrossRef]
- Kotsyuba, E.P. Effect of elevated temperature and of hypoxia on NO activity in the central nervous system of bivalve molluscs. J. Evol. Biochem. Physiol. 2008, 44, 237–246. [Google Scholar] [CrossRef]
- Tielens, A.G.; Rotte, C.; van Hellemond, J.J.; Martin, W. Mitochondria as we don’t know them. Trends Biochem. Sci. 2002, 27, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Hochachka, P.W. Defense Strategies against Hypoxia and Hypothermia. Science 1986, 231, 234–241. [Google Scholar] [CrossRef]
- Ballantyne, J.S. Mitochondria: Aerobic and anaerobic design—Lessons from molluscs and fishes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 461–467. [Google Scholar] [CrossRef]
- David, E.; Tanguy, A.; Pichavant, K.; Moraga, D. Response of the Pacific oyster Crassostrea gigas to hypoxia exposure under experimental conditions. FEBS J. 2005, 272, 5635–5652. [Google Scholar] [CrossRef] [PubMed]
- Boveris, A.; Arnaiz, S.L.; Alvarez, S.; Costa, L.E.; Valdez, L. The mitochondrial production of free radicals. In Free Radicals in Chemistry, Biology and Medicine; Yoshikawa, T., Toyokuni, S., Yamamoto, Y., Naito, Y., Eds.; Oica International: London, UK, 2000. [Google Scholar]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Brown, G.C.; Borutaite, V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free. Radic. Biol. Med. 2002, 33, 1440–1450. [Google Scholar] [CrossRef]
- Abele, D.; Kruppe, M.; Philipp, E.E.R.; Brey, T. Mantle cavity water oxygen partial pressure (Po2) in marine molluscs aligns with lifestyle. Can. J. Fish. Aquat. Sci. 2010, 67, 977–986. [Google Scholar] [CrossRef] [Green Version]
- Strahl, J.; Brey, T.; Philipp, E.E.R.; Thorarinsdóttir, G.; Fischer, N.; Wessels, W.; Abele, D. Physiological responses to self-induced burrowing and metabolic rate depression in the ocean quahog Arctica islandica. J. Exp. Biol. 2011, 214, 4223–4233. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D.R. Origins and Evolution of Pathways of Anaerobic Metabolism in the Animal Kingdom. Integr. Comp. Biol. 1991, 31, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Regulation of Mammalian O2 Homeostasis by Hypoxia-Inducible Factor 1. Annu. Rev. Cell Dev. Biol. 1999, 15, 551–578. [Google Scholar] [CrossRef]
- Semenza, G.L. Signal transduction to hypoxia-inducible factor 1. Biochem. Pharmacol. 2002, 64, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor. Exp. Physiol. 2006, 91, 803–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-W.; Xiao, S.-S.; Zhang, R.; Liu, L.; Zhu, H. Physiological changes and transcriptional modulation of HIF-αs in Siberian sturgeon in response to hypoxia. Aquaculture 2021, 545, 737219. [Google Scholar] [CrossRef]
- Dolt, K.S.; Mishra, M.K.; Karar, J.; Baig, M.A.; Ahmed, Z.; Pasha, M.Q. cDNA cloning, gene organization and variant specific expression of HIF-1α in high altitude yak (Bos grunniens). Gene 2007, 386, 73–80. [Google Scholar] [CrossRef]
- Loenarz, C.; Coleman, M.L.; Boleininger, A.; Schierwater, B.; Holland, P.W.; Ratcliffe, P.J.; Schofield, C.J. The hypoxia inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens. EMBO Rep. 2011, 12, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [Green Version]
- Heerden, D.; Vosloo, A.; Nikinmaa, M. Effects of short-term copper exposure on gill structure, metallothionein and hypoxia-inducible factor-1α (HIF-1α) levels in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2004, 69, 271–280. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Kallio, P.J.; Okamoto, K.; O’Brien, S.; Carrero, P.; Makino, Y.; Tanaka, H.; Poellinger, L. Signal transduction in hypoxic cells: Inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 1998, 17, 6573–6586. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.-W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Epstein, A.C.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A.; et al. C. elegans EGL-9 and Mammalian Homologs Define a Family of Dioxygenases that Regulate HIF by Prolyl Hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.H.; Zheng, J.Z.; Leung, S.W.; Roe, R.; Semenza, G.L. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha: Modulation of transcriptional activity by oxygen tension. J. Biol. Chem. 1997, 272, 19253–19260. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Nettleton, D.; Jiang, M.; Kim, S.K.; Powell-Coffman, J.A. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J. Biol. Chem. 2005, 280, 20580–20588. [Google Scholar] [CrossRef] [Green Version]
- Piontkivska, H.; Chung, J.S.; Ivanina, A.V.; Sokolov, E.P.; Techa, S.; Sokolova, I.M. Molecular characterization and mRNA expression of two key enzymes of hypoxia-sensing pathways in eastern oysters Crassostrea virginica (Gmelin): Hypoxia-inducible factor α (HIF-α) and HIF-prolyl hydroxylase (PHD). Comp. Biochem. Physiol. Part D Genom. Proteom. 2011, 6, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Kawabe, S.; Yokoyama, Y. Role of Hypoxia-Inducible Factor α in Response to Hypoxia and Heat Shock in the Pacific Oyster Crassostrea gigas. Mar. Biotechnol. 2012, 14, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shin, P.; Cheung, S. Isolation and mRNA expression of hypoxia-inducible factor α (HIF-α) in two sublittoral nassariid gastropods: Nassarius siquijorensis and Nassarius conoidalis. Mar. Environ. Res. 2014, 99, 44–51. [Google Scholar] [CrossRef]
- Cai, X.; Huang, Y.; Zhang, X.; Wang, S.; Zou, Z.; Wang, G.; Wang, Y.; Zhang, Z. Cloning, characterization, hypoxia and heat shock response of hypoxia inducible factor-1 (HIF-1) from the small abalone Haliotis diversicolor. Gene 2014, 534, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Li, W.; Liang, X.; Liu, J.; Ge, H.; Dong, Z. Unravelling the characterization of hypoxia-inducible factor-1α (HIF-1α) and antioxidant enzymes in clam Cyclina sinensis in response to hypoxia. Aquac. Res. 2022, 53, 5937–5945. [Google Scholar] [CrossRef]
- Rytkönen, K.T.; Williams, T.; Renshaw, G.M.; Primmer, C.; Nikinmaa, M. Molecular Evolution of the Metazoan PHD–HIF Oxygen-Sensing System. Mol. Biol. Evol. 2011, 28, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Jiang, K.; Zhang, F.; Song, W.; Zhao, M.; Meng, Y.; Chen, F.; Zhao, M.; Ma, L. Two transcripts of hypoxia inducible factor-1 (HIF-1) from Scylla paramamosain Estampador, 1950 (Brachyura: Portunidae) and their expression profiles under different hypoxic conditions. J. Crustac. Biol. 2017, 37, 45–52. [Google Scholar] [CrossRef] [Green Version]
- McMahon, B.R. Respiratory and circulatory compensation to hypoxia in crustaceans. Respir. Physiol. 2001, 128, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001, 13, 167–171. [Google Scholar] [CrossRef]
- Nikinmaa, M.; Ress, B.B. Oxygen-dependent gene expression in fishes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1079–R1090. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Park, C.; Kim, E.; Nam, Y. Transcriptional modulation patterns of abalone Haliotis discus hannai hypoxia inducible factor-1α (HIF-1α) in interdependent crosstalk between hypoxia, infection, and environmental stresses. Aquac. Rep. 2021, 19, 100566. [Google Scholar] [CrossRef]
- Camacho-Jiménez, L.; Peregrino-Uriarte, A.; Martínez-Quintana, J.; Yepiz-Plascencia, G. The glyceraldehyde-3-phosphate dehydrogenase of the shrimp Litopenaeus vannamei: Molecular cloning, characterization and expression during hypoxia. Mar. Environ. Res. 2018, 138, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.P.; Lucu, C.; Onken, H.; Weihrauch, D. Multiple functions of the crustacean gill: Osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front. Physiol. 2012, 3, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, P., Jr.; Mcmullen, D.C.; Storey, K.B. Hif-1alpha involvement in low temperature and anoxia survival by a freeze tolerant Insect. Mol. Cell. Biochem. 2005, 280, 99–106. [Google Scholar] [CrossRef]
- Kotsyuba, E.P. Hypoxia-inducible factor 1α in the central nervous system of the scallop Mizuhopecten yessoensis Jay, 1857 (Bivalvia: Pectinidae) during anoxia and elevated temperatures. Russ. J. Mar. Biol. 2017, 43, 293–301. [Google Scholar] [CrossRef]
- Jadhav, M.; Gulave, A.; Bawane, V. Role of cerebral ganglia in regulation of oxygen consumption of freshwater bivalve mollusc, Lamellidens marginalis from Godavari River during summer season. Biosci. Discov. 2012, 3, 337–341. [Google Scholar]
- Mane, U.H.; Rao, K.R.; Muley, S.D.; Vedpathak, A.N. Probable role of nerve ganglia in respiration of the estuarine clam, Katelysia opima. Indian J. Comp. Anim. Physiol. 1990, 8, 21–27. [Google Scholar]
- Vedpathak, A.N.; Jadhav, M.R.; Misalk, P. Role of cerebral ganglia in regulation of oxygen consumption of freshwater bivalve mollusc, Indonaia caeruleus (Prashad, 1918) from Godavari river during summer. Bioscan 2011, 6, 609–612. [Google Scholar]
- Motavkine, P.A.; Varaksine, A.A. Le reproduction chez lez mollusques bivalves role du systeme nervaux et regulation. In Rapports Scientifiques et Techniques; IFREMER: Brest, France, 1989; Volume 10, pp. 1–250. [Google Scholar]
- Stroka, D.M.; Burkhardt, T.; Desbaillets, I.; Wenger, R.H.; Neil, D.A.H.; Bauer, C.; Gassmann, M.; Candinas, D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001, 15, 2445–2453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiq, A.; Ayoub, I.A.; Chavez, J.C.; Aminova, L.; Shah, S.; LaManna, J.C.; Patton, S.M.; Connor, J.R.; Cherny, R.A.; Volitakis, I.; et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition: A target for neuroprotection in the central nervous system. J. Biol. Chem. 2005, 280, 41732–41743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Hernández, B.; Posadas, I.; Podlesniy, P.; Abad, M.A.; Trullas, R.; Ceña, V. HIF-1α is neuroprotective during the early phases of mild hypoxia in rat cortical neurons. Exp. Neurol. 2012, 233, 543–544. [Google Scholar] [CrossRef]
- Vangeison, G.; Carr, D.; Federoff, H.J.; Rempe, D.A. The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1alpha in neurons and astrocytes. J. Neurosci. 2008, 28, 1988–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarember, K.A.; Malech, H.L. HIF-1alpha: A master regulator of innate host defenses? J. Clin. Investig. 2005, 115, 1702–1704. [Google Scholar] [CrossRef]
- Formenti, F.; Constantin-Teodosiu, D.; Emmanuel, Y.; Cheeseman, J.; Dorrington, K.L.; Edwards, L.M.; Humphreys, S.M.; Lappin, T.R.J.; McMullin, M.F.; McNamara, C.J.; et al. Regulation of human metabolism by hypoxia-inducible factor. Proc. Natl. Acad. Sci. USA 2010, 107, 12722–12727. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.J.; Bargmann, C.I. Hypoxia and the HIF-1 transcriptional pathway reorganize a neuronal circuit for oxygen-dependent behavior in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2008, 105, 7321–7326. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Lannig, G. Chapter 4. Oxygen and Capacity Limited Thermal Tolerance. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2009; Volume 27, pp. 143–191. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsyuba, E.; Dyachuk, V. Role of the Neuroendocrine System of Marine Bivalves in Their Response to Hypoxia. Int. J. Mol. Sci. 2023, 24, 1202. https://doi.org/10.3390/ijms24021202
Kotsyuba E, Dyachuk V. Role of the Neuroendocrine System of Marine Bivalves in Their Response to Hypoxia. International Journal of Molecular Sciences. 2023; 24(2):1202. https://doi.org/10.3390/ijms24021202
Chicago/Turabian StyleKotsyuba, Elena, and Vyacheslav Dyachuk. 2023. "Role of the Neuroendocrine System of Marine Bivalves in Their Response to Hypoxia" International Journal of Molecular Sciences 24, no. 2: 1202. https://doi.org/10.3390/ijms24021202
APA StyleKotsyuba, E., & Dyachuk, V. (2023). Role of the Neuroendocrine System of Marine Bivalves in Their Response to Hypoxia. International Journal of Molecular Sciences, 24(2), 1202. https://doi.org/10.3390/ijms24021202