Fabrication and Characterization Techniques of In Vitro 3D Tissue Models
Abstract
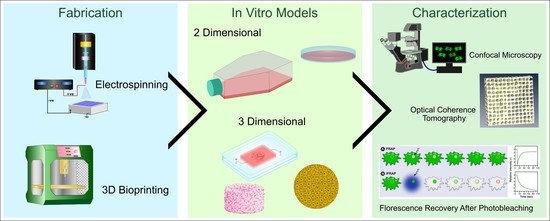
:1. Introduction
2. Types of 3D Tissue Models
2.1. Anchorage Independent (Non-Scaffold Based) 3D Tissue Models
2.1.1. Spheroids
2.1.2. Organoids
2.1.3. Cell Sheet Engineering
2.2. Anchorage Dependent (Scaffold Based) 3D Tissue Models
2.2.1. Solvent Casting Particulate Leaching (SCPL)
2.2.2. Electrospinning
2.2.3. Bioprinting
2.2.4. Organ-on-a-Chip
3. Biomaterials for 3D Tissue Modelling
| Biomaterial | Type | Pros | Cons | Ref |
|---|---|---|---|---|
| Collagen | Natural | High biocompatibility, biodegradable, high cell adhesion, and cell remodeling. Has high printability, is biocompatible, low immunogenicity | Poor mechanical properties, unpredictable degradation in vivo, high thrombogenic potential | [87] |
| Gelatin | Natural | Cheap, biocompatible, easy to modify, good proliferation, biodegradable | Brittleness, low mechanical properties, fast degradation | [88] |
| Chitosan | Natural | Biocompatible, biodegradable, high cell proliferation | Lower mechanical properties, immunogenic | [89] |
| Fibrin | Natural | High cell adhesion and viability, quick gelation and good cell migration, and vascularization | low printability, biocompatibility, low mechanical strength | [90] |
| Hyaluronic Acid | Natural | Biocompatible, biodegradable, high cell proliferation and viability, high printability | low mechanical strength | [91] |
| Alginate | Natural | Biocompatible, biodegradable, sustained release, adoptable mechanical strength with cell growth, rapid gelation | low cell adhesion | [92] |
| Pectin | Natural | Cheap, biocompatible, can be modified, plant derived, good cell proliferation, biodegradable | Poor mechanical properties, Slower gelation time | [93] |
| Decellularized ECM | Natural | Keeps vasculature network intact | Variation caused by different decellularization methods, | [94] |
| Starch | Natural | Cheap, biocompatible, versatile rheology, | Poor mechanical properties, slower gelation time, needs high temperature (70–90 °C) to gelatinize, at higher temperatures, phase separation between composite materials may occur | [95] |
| Fucoidan | Natural | Good bioactive properties, biocompatible, biodegradable, used to enhance properties of other natural biomaterials | Does not gel on its own, crosslinking strategies need to be optimized, high synthesis cost | [96] |
| Silk Fibroin | Natural | Biocompatible, good mechanical properties | High cost of production, | [97] |
| Hydroxyapatite | Natural/Synthetic Synthesis | Bioactive, biocompatible, hydrophilic, | brittleness, low tensile strength and fracture toughness | [98,99] |
| Polycaprolactone (PCL) | Synthetic | Moderate mechanical strength. Biocompatible | Slow degradation, lower cell adhesion/aggregation, hydrophobic, inflammation due to acid degradation products | [100] |
| Poly Lactic-co-Glycolic Acid (PLGA) | Synthetic | Biocompatible, biodegradable, immunogenic | Brittle and relatively hard, lower cell adhesion/aggregation, inflammation due to acid degradation products | [101] |
| Poly(itaconate-co-citrate-cooctanediol) (PICO) | Synthetic | Biocompatible, biodegradable, cheap, good mechanical properties, fast crosslinking, non-cytotoxic to cells | UV cross linking | [102] |
| Poly (ethylene glycol) (PEG) | Synthetic | Biocompatible, biodegradable, can be modified with various functional groups | Moderate mechanical strength, low printability, difficulty in scalability, Lower cell adhesion | [92] |
| Polyphosphazenes | Synthetic | Biocompatible, good mechanical properties, slow degradation (hard tissues) | Slow degradation (soft tissues) | [97,103] |
| Polyurethanes | Synthetic | Good mechanical properties, good rheological properties | Poor degradability, copolymerization is required | [104] |
| Polyanhydrides | Synthetic | Good flexibility, controllable degradation rates | Weak mechanical properties | [104] |
| Poly(propelene-fumarate) | Synthetic | Good processability, good ductility, biocompatibility, easily forms covalent polymer networks | Challenging to handle the material due to high viscosity, increased cytotoxicity and acute inflammation, variation in molecular weight between crosslinking agents | [105,106] |
| Metals | Synthetic | Biocompatible with good mechanical properties, low degradability (Tissue dependent) | Subject to oxidation, low degradability (Tissue dependent), may be cytotoxic due to release of free metal ions | [107] |
| Ceramics | Synthetic | Osteoinductive and osteoconductive in bioactive ceramics, low toxicity, biocompatible, angiogenetic potential, | High brittleness, weak, low bioactivity | [107] |
Characterization and Optimization of Biomaterials
4. Cell Sources
5. Imaging Modalities of 3D Tissue Models
| Imaging Modalities | Characteristics | Application | Ref |
|---|---|---|---|
| Fluorescence | Cells are marked with fluorescence markers and a sample is irradiated with wavelengths between visible and ultraviolet to reveal fluorescent species. | Cell viability, proliferation | [119] |
| Confocal Imaging | Advanced version of fluorescence resulting in high-resolution images by collecting light from a single plane of focus and eliminating out-of-focus light | Cellular Structure, viability, live imaging, 3D reconstruction | [120] |
| Scanning Electron Microscope (SEM) | A technique used to produce high-resolution images of surface topography by scanning with electrons on the surface | Surface morphology | [121] |
| Transmission Electron Microscope (TEM) | A technique in which electrons pass through ultrathin samples to generate high-resolution images | Characterization of pore structure, nano structures | [122] |
| Fluorescence Recovery After Photobleaching (FRAP) | A high-intensity laser causes bleaching in a region of interest (ROI) and gradual recovery of fluorescence from the surrounding environment to the bleached area is observed. | Used to study oxygen and nutrient diffusion across cells and tissue structures. | [119] |
| Fluorescence Loss in Photobleaching (FLIP) | Involves repeated bleaching of an ROI and measuring fluorescence intensity outside the bleached area where a drop in fluorescence intensity due to bleached non-fluorescent molecules provides quantitative data on molecular mobility. | Molecular mobility, exchange of molecules between cell compartments | [123] |
| Fluorescence localization after photobleaching (FLAP) | Involves labeling molecules with two fluorescent labels: one to be bleached locally and the second is a reference label that remains intact. By measuring the difference between bleached and unbleached signals gives an absolute FLAP signal which can be used to track the labeled molecule. | Ability to identify molecules and populations that have varying speeds and have dissimilar dynamics. | [123] |
| Fluorescence Resonance Energy Transfer (FRET) | A physical process in which a molecular fluorophore is excited and a nonradiative energy transfer occurs to another fluorophore through intermolecular long-range dipole-dipole coupling. This process is highly dependent on the distance between two fluorophores. | Live -cell analysis of cell biology, cellular interaction in 3D scaffolds, Protein–protein interaction, receptor activation, intramolecular distances | [124,125] |
| Fluorescence Lifetime Imaging Microscopy (FLIM) | A method in which the fluorescence decay time is measured. In combination with FRET, this method can be used to map spatial distribution to indirectly measure bimolecular interactions, concentration, and conformational changes. | Measuring intramolecular distances, evaluate therapeutic efficacy in drug screening | [119] |
| Phosphorescence Lifetime Imaging Microscopy (PLIM) | Similar to FLIM; however, this process images phosphorescence quenching. | Measuring partial oxygen concentration and identify hypoxic environment. | [119] |
| Optical Coherence Tomography (OCT) | Measures optical backscatter from different microstructural features within materials and tissues to generate high-resolution images of the cross-sections of tissue. | Quantify changes in porosity of scaffold, pore size, pore interconnectivity, cell dynamics and tissue development. | [126,127] |
| Micro-Computerized Tomography (MCT) | Involves exploiting variations in X-ray absorption, refraction, and scattering to form contrast alterations resulting in spatial distribution of material densities and providing 3D images of the internal structure. | Largely used in bone tissue models, as this technique offers the ability to form contrasts between soft and hard tissues. | [119] |

5.1. Fluorescence Recovery after Photobleaching (FRAP) Using Confocal Microscopy
5.2. Optical Coherence Tomography (OCT)
6. Other Imaging Modalities
7. Conclusions and Future Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duval, K.; Grover, H.; Han, L.; Mou, Y.; Pegoraro, A.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [Green Version]
- Edmondson, R.; Broglie, J.; Adcock, A.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 32, 266–277. [Google Scholar] [CrossRef] [Green Version]
- Langhans, S. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Kusindarta, D.; Wihadmadyatami, H. The Role of Extracellular Matrix in Tissue Regeneration. In Tissue Regeneration; InTech: Singapore, 2018. [Google Scholar] [CrossRef] [Green Version]
- Gibot, L. 3D tissue models to bridge the gap between cell culture and tissue in assessing electroporation. In Handbook of Electroporation; Springer: Cham, Germany, 2017. [Google Scholar] [CrossRef]
- Ruan, J.; Tulloch, N.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Di Silvio, L. Bone tissue engineering and biomineralization. In Tissue Engineering Using Ceramics and Polymers; Elsevier Ltd.: Amsterdam, The Netherlands, 2007; pp. 319–331. [Google Scholar] [CrossRef]
- Torras, N.; García-Díaz, M.; Fernández-Majada, V.; Martínez, E. Mimicking epithelial tissues in three-dimensional cell culture models. Front. Bioeng. Biotechnol. 2018, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Oberman, R.; Bhardwaj, A. Physiology, Cardiac; StatPearls Publishing: Vienna, Austria, 2019. [Google Scholar]
- Omar, A.; Vallabhajosyula, S.; Sengupta, P. Left Ventricular Twist and Torsion. Circ. Cardiovasc. Imaging 2015, 8, e003029. [Google Scholar] [CrossRef] [Green Version]
- Baker, B.; Chen, C. Deconstructing the third dimension-how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.; Anuradha, E.; Solomon, F.P. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Dhaliwal, A. Three Dimensional Cell Culture: A Review. Mater. Methods 2012, 2. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Griffanti, G.; Rezabeigi, E.; Li, J.; Murshed, M.; Nazhat, S. Rapid Biofabrication of Printable Dense Collagen Bioinks of Tunable Properties. Adv. Funct. Mater. 2020, 30, 1903874. [Google Scholar] [CrossRef]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Eglen, R. Three-Dimensional Cell Cultures in Drug Discovery and Development. Adv. Sci. Drug Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.; Moreira, A.; de Melo-Diogo, D.; Gaspar, V.; Carvalho, M.; Correia, I. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- De Hoogt, R.; Estrada, M.; Vidic, S.; Davies, E.; Osswald, A.; Barbier, M.; Santo, V.; Gjerde, K.; Van Zoggel, H.; Blom, S.; et al. Data descriptor: Protocols and characterization data for 2d, 3d, and slice-based tumor models from the predect project. Sci. Data 2017, 4, 170170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, R.; McCredie, J.; Inch, W. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J. Natl. Cancer Inst. 1971, 46, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; De Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ryu, N.; Lee, S.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, W.; Park, S. A microfluidic spheroid culture device with a concentration gradient generator for high-throughput screening of drug efficacy. Molecules 2018, 23, 3355. [Google Scholar] [CrossRef] [Green Version]
- Utama, R.; Atapattu, L.; O’Mahony, A.; Fife, C.; Baek, J.; Allard, T.; O’Mahony, K.; Ribeiro, J.; Gaus, K.; Kavallaris, M.; et al. A 3D Bioprinter Specifically Designed for the High-Throughput Production of Matrix-Embedded Multicellular Spheroids. IScience 2020, 23, 101621. [Google Scholar] [CrossRef] [PubMed]
- Moshksayan, K.; Kashaninejad, N.; Warkiani, M.; Lock, J.; Moghadas, H.; Firoozabadi, B.; Saidi, M.; Nguyen, N. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens. Actuators B Chem. 2018, 263, 151–176. [Google Scholar] [CrossRef] [Green Version]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and animal models: Are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benien, P.; Swami, A. 3D tumor models: History, advances and future perspectives. Futur. Oncol. 2014, 10, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Davies, J. Organoids and mini-organs: Introduction, history, and potential. In Organoids and Mini-Organs; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 3–23. [Google Scholar] [CrossRef]
- Augustyniak; Bertero, A.; Coccini, T.; Baderna, D.; Buzanska, L.; Caloni, F. Organoids are promising tools for species-specific in vitro toxicological studies. J. Appl. Toxicol. 2019, 39, 1610–1622. [Google Scholar] [CrossRef]
- Velasco, V.; Shariati, S.; Esfandyarpour, R. Microtechnology-based methods for organoid models. Microsyst Nanoeng. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472, 51–56. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.; Ueno, Y.; Zheng, Y.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Lancaster, M.; Knoblich, J. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.; Dye, B.; Ferrer-Torres, D.; Hill, D.; Overeem, A.; Shea, L.; Spence, J. Generation of lung organoids from human pluripotent stem cells in vitro. Nat. Protoc. 2019, 14, 518–540. [Google Scholar] [CrossRef]
- Richards, D.; Li, Y.; Kerr, C.; Yao, J.; Beeson, G.; Coyle, R.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, C.; Wu, Y.-H.; Liu, L.-B.; Zhen, Z.-D.; Fan, D.-Y.; Song, Z.-R.; Chang, J.-T.; Wang, P.-G.; An, J. Mice 3D testicular organoid system as a novel tool to study Zika virus pathogenesis. Virol. Sin. 2022; in press. [Google Scholar] [CrossRef]
- Joseph, J.S.; Malindisa, S.T.; Ntwasa, M. Two-Dimensional (2D) and Three-Dimensional (3D) Cell Culturing in Drug Discovery. In Cell Culture; IntechOpen: Singapore, 2019. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Yeo, M.; Yang, G.; Kim, G. Cell-electrospinning and its application for tissue engineering. Int. J. Mol. Sci. 2019, 20, 6208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend-Nicholson, A.; Jayasinghe, S. Cell electrospinning: A unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules 2006, 7, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, H.; Wang, S.; Li, L.; Wang, R.; Jiang, S. Human umbilical cord-derived stem cell sheets improve left ventricular function in rat models of ischemic heart failure. Eur. J. Pharmacol. 2022, 925, 174994. [Google Scholar] [CrossRef] [PubMed]
- Owaki, T.; Shimizu, T.; Yamato, M.; Okano, T. Cell sheet engineering for regenerative medicine: Current challenges and strategies. Biotechnol. J. 2014, 9, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Chang, H. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Tocchi, A.; Holly, M.; Parks, W.; Smith, J. A small intestinal organoid model of non-invasive enteric pathogen-epithelial cell interactions. Mucosal Immunol. 2015, 8, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Evans, D. Tissue Engineering: The Future of Stem Cells. Top. Tissue Eng. 2005, 2, 1–21. [Google Scholar]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2019, 96, 153–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Xie, M.B.; Li, Y.; Ma, Y.; Li, J.; Dai, F. Recent progress in tissue engineering and regenerative medicine. J. Biomater. Tissue Eng. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.; García-Aznar, J.; Doblaré, M. On scaffold designing for bone regeneration: A computational multiscale approach. Acta Biomater. 2009, 5, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Brougham, C.; Levingstone, T.; Shen, N.; Cooney, G.; Jockenhoevel, S.; Flanagan, T.; O’Brien, F. Freeze-Drying as a Novel Biofabrication Method for Achieving a Controlled Microarchitecture within Large, Complex Natural Biomaterial Scaffolds. Adv. Healthc. Mater. 2017, 6, 1700598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anandan, D.; Stella, S.M.; Nambiraj, N.A.; Vijayalakshmi, U.; Jaiswal, A. Development of mechanically compliant 3D composite scaffolds for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2018, 106, 3267–3274. [Google Scholar] [CrossRef]
- Martínez-Pérez, C.A.; Olivas-Armendariz, I.; Castro-Carmona, J.S.; García-Casillas, P.E. Scaffolds for Tissue Engineering Via Thermally Induced Phase Separation. In Advances in Regenerative Medicine; IntechOpen: Singapore, 2011. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, F.; Annabi, N. Engineering porous scaffolds using gas-based techniques. Curr. Opin. Biotechnol. 2011, 22, 661–666. [Google Scholar] [CrossRef]
- Zhong, W. Nanofibres for Medical Textiles. In Advances in Smart Medical Textiles: Treatments and Health Monitoring; Woodhead Publishing: Southen, UK, 2016. [Google Scholar] [CrossRef]
- Zheng, Y. Fabrication on bioinspired surfaces. In Bioinspired Design of Materials Surfaces; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Singh, R.; Eitler, D.; Morelle, R.; Friedrich, R.; Dietel, B.; Alexiou, C.; Boccaccini, A.; Liverani, L.; Cicha, I. Optimization of cell seeding on electrospun PCL-silk fibroin scaffolds. Eur. Polym. J. 2020, 134, 109838. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mirdamadi, E.; Tashman, J.; Shiwarski, D.; Palchesko, R.; Feinberg, A. FRESH 3D bioprinting a full-size model of the human heart. ACS Biomater. Sci. Eng. 2020, 6, 6453–6459. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Senior, J.; Cooke, M.; Grover, L.; Smith, A.; Senior, J.; Smith, A.; Cooke, M.; Grover, L. Fabrication of Complex Hydrogel Structures Using Suspended Layer Additive Manufacturing (SLAM). Adv. Funct. Mater. 2019, 29, 1904845. [Google Scholar] [CrossRef] [Green Version]
- Skylar-Scott, M.; Uzel, S.; Nam, L.; Ahrens, J.; Truby, R.; Damaraju, S.; Lewis, J. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2019, 5, eaaw2459. [Google Scholar] [CrossRef]
- Burdis, R.; Kelly, D. Biofabrication and bioprinting using cellular aggregates, microtissues and organoids for the engineering of musculoskeletal tissues. Acta Biomater. 2021, 126, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.; Fukunishi, T.; Zhang, H.; Huang, C.; Nashed, A.; Blazeski, A.; Disilvestre, D.; Vricella, L.; Conte, J.; Tung, L.; et al. Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci. Rep. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [Green Version]
- Loai, S.; Kingston, B.R.; Wang, Z.; Philpott, D.N.; Tao, M.; Cheng, H.-L.M. Clinical Perspectives on 3D Bioprinting Paradigms for Regenerative Medicine. Regen. Med. Front. 2019, 1, e190004. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Aziz, A.; Geng, C.; Fu, M.; Yu, X.; Qin, K.; Liu, B. The role of microfluidics for organ on chip simulations. Bioengineering 2017, 4, 39. [Google Scholar] [CrossRef]
- Zhao, Y.; Rafatian, N.; Feric, N.; Cox, B.; Aschar-Sobbi, R.; Wang, E.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 2019, 176, 913.e18–927.e18. [Google Scholar] [CrossRef] [Green Version]
- Parsa, H.; Wang, B.; Vunjak-Novakovic, G. A microfluidic platform for the high-throughput study of pathological cardiac hypertrophy. Lab Chip 2017, 17, 3264–3271. [Google Scholar] [CrossRef]
- Zhao, Y.; Rafatian, N.; Wang, E.; Feric, N.; Lai, B.; Knee-Walden, E.; Backx, P.; Radisic, M. Engineering microenvironment for human cardiac tissue assembly in heart-on-a-chip platform. Matrix Biol. 2019, 85-86, 189–204. [Google Scholar] [CrossRef]
- Jang, K.; Suh, K. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 2010, 10, 36–42. [Google Scholar] [CrossRef]
- Kilic, O.; Pamies, D.; Lavell, E.; Schiapparelli, P.; Feng, Y.; Hartung, T.; Bal-Price, A.; Hogberg, H.; Quinones-Hinojosa, A.; Guerrero-Cazares, H.A. Levchenko, Brain-on-a-chip model enables analysis of human neuronal differentiation and chemotaxis. Lab Chip 2016, 16, 4152–4162. [Google Scholar] [CrossRef] [Green Version]
- Grigoryan, B.; Paulsen, S.; Corbett, D.; Sazer, D.; Fortin, C.; Zaita, A.; Greenfield, P.; Calafat, N.; Gounley, J.; Ta, A.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Am. Assoc. Adv. Sci. 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Sontheimer-Phelps, A.; Chou, D.; Tovaglieri, A.; Ferrante, T.; Duckworth, T.; Fadel, C.; Frismantas, V.; Sutherland, A.; Jalili-Firoozinezhad, S.; Kasendra, M.; et al. Human Colon-on-a-Chip Enables Continuous In Vitro Analysis of Colon Mucus Layer Accumulation and Physiology. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 507–526. [Google Scholar] [CrossRef] [Green Version]
- Bhise, N.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.; Shin, S.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.; Byun, W.; Alisafaei, F.; Georgescu, A.; Yi, Y.; Massaro-Giordano, M.; Shenoy, V.; Lee, V.; Bunya, V.; Huh, D. Multiscale reverse engineering of the human ocular surface. Nat. Med. 2019, 25, 1310–1318. [Google Scholar] [CrossRef]
- Costa, P.; Albers, H.; Linssen, J.; Middelkamp, H.; Van Der Hout, L.; Passier, R.; Van Den Berg, A.; Malda, J.; Van Der Meer, A. Mimicking arterial thrombosis in a 3D-printed microfluidic: In vitro vascular model based on computed tomography angiography data. Lab Chip 2017, 17, 2785–2792. [Google Scholar] [CrossRef] [Green Version]
- Lan, D.; Shang, Y.; Su, H.; Liang, M.; Liu, Y.; Li, H.; Feng, Q.; Cao, X.; Dong, H. Facile Fabrication of Hollow Hydrogel Microfiber via 3D Printing-Assisted Microfluidics and Its Application as a Biomimetic Blood Capillary. ACS Biomater. Sci. Eng. 2021, 7, 4971–4981. [Google Scholar] [CrossRef]
- Hong, S.; Song, J. 3D bioprinted drug-resistant breast cancer spheroids for quantitative in situ evaluation of drug resistance. Acta Biomater. 2022, 138, 228–239. [Google Scholar] [CrossRef] [PubMed]
- van Meer, B.; de Vries, H.; Firth, K.; van Weerd, J.; Tertoolen, L.; Karperien, H.; Jonkheijm, P.; Denning, C.; IJzerman, A.; Mummery, C. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.; Wu, Q.; Yazbeck, J.; Liu, C.; Okhovatian, S.; Radisic, M. Beyond polydimethylsiloxane: Alternative materials for fabrication of organ on a chip devices and microphysiological systems. ACS Biomater. Sci. Eng. 2020, 7, 2880–2899. [Google Scholar] [CrossRef] [PubMed]
- Abaci, H.; Shuler, M. Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr. Biol. 2015, 7, 383–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Ko, H.; Kwon, I.; Shin, K. Extracellular matrix revisited: Roles in tissue engineering. Int. Neurourol. J. 2016, 20, S23–S29. [Google Scholar] [CrossRef] [Green Version]
- NIH Stem Cell, NIH Stem Cell Information Home Page—Stem Cell Basics, In Stem Cell Information. Available online: https://stemcells.nih.gov/ (accessed on 31 December 2022).
- Doss, M.; Sachinidis, A. Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Ricklefs, M.; Korossis, S.; Haverich, A.; Schilling, T. Polymeric Scaffolds for Bioartificial Cardiovascular Prostheses. In Scaffolds in Tissue Engineering—Materials, Technologies and Clinical Applications; IntechOpen: Singapore, 2017. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Li, Y.; Deng, B.; Liu, X.; Wang, L.; Zhu, Q. Chitosan hydrogel improves mesenchymal stem cell transplant survival and cardiac function following myocardial infarction in rats. Exp. Ther. Med. 2017, 13, 588–594. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Lee, S.; Cheng, H.; Yoo, J.; Atala, A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef]
- Gaetani, R.; Doevendans, P.; Metz, C.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33, 1782–1790. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef] [Green Version]
- Mehrali, M.; Thakur, A.; Kadumudi, F.; Pierchala, M.; Cordova, J.; Shahbazi, M.; Mehrali, M.; Pennisi, C.; Orive, G.; Gaharwar, A.; et al. Pectin Methacrylate (PEMA) and Gelatin-Based Hydrogels for Cell Delivery: Converting Waste Materials into Biomaterials. ACS Appl. Mater. Interfaces 2019, 11, 12283–12297. [Google Scholar] [CrossRef]
- Kc, P.; Hong, Y.; Zhang, G. Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair: Advantages and challenges. Regen. Biomater. 2019, 6, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Li, J.; Cui, M.; Wang, J.; Zhou, Y.; Luo, L.; Wei, Y.; Ye, L.; Sun, H.; Yao, F. In Situ “clickable” Zwitterionic Starch-Based Hydrogel for 3D Cell Encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 4442–4455. [Google Scholar] [CrossRef]
- Reys, L.; Silva, S.; Da Costa, D.S.; Oliveira, N.; Mano, J.; Reis, R.; Silva, T. Fucoidan Hydrogels Photo-Cross-Linked with Visible Radiation As Matrices for Cell Culture. ACS Biomater. Sci. Eng. 2016, 2, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, C.; Thouas, G.A. Progress and challenges in biomaterials used for bone tissue engineering: Bioactive glasses and elastomeric composites. Prog. Biomater. 2012, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Family, R.; Solati-Hashjin, M.; Nik, S.; Nemati, A. Surface modification for titanium implants by hydroxyapatite nanocomposite. Casp. J. Intern. Med. 2012, 3, 460. [Google Scholar]
- Lee, H.; Byun, S.; Cho, S.; Yang, B. Past, present, and future of regeneration therapy in oral and periodontal tissue: A review. Appl. Sci. 2019, 9, 1046. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.; Mishra, A.; Lin, P.; Ng, S.; Yeong, W.; Kim, Y.; Yoon, Y. 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol. Biosci. 2017, 17, 1600250. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.V.; Grigoryev, A.; Krotova, L.; Skaletsky, N.; Popov, V.; Sevastianov, V. 3D printing of PLGA scaffolds for tissue engineering. J. Biomed. Mater. Res. A 2017, 105, 104–109. [Google Scholar] [CrossRef]
- Savoji, H.; Huyer, L.D.; Mohammadi, M.; Lai, B.L.; Rafatian, N.; Bannerman, D.; Shoaib, M.; Bobicki, E.; Ramachandran, A.; Radisic, M. 3D Printing of Vascular Tubes Using Bioelastomer Prepolymers by Freeform Reversible Embedding. ACS Biomater. Sci. Eng. 2020, 6, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.; Nair, L.; Laurencin, C. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunduru, K.; Basu, A.; Domb, A. Biodegradable Polymers: Medical Applications. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Kasper, F.; Tanahashi, K.; Fisher, J.; Mikos, A. Synthesis of poly(propylene fumarate). Nat. Protoc. 2009, 4, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Kinard, L.; Kasper, F.; Mikos, A. Synthesis of oligo(Poly(ethylene glycol) fumarate). Nat. Protoc. 2012, 7, 1219–1227. [Google Scholar] [CrossRef] [Green Version]
- Bahraminasab, M.; Sahari, B.; Edwards, K.; Farahmand, F.; Arumugam, M. Aseptic loosening of femoral components—Materials engineering and design considerations. Mater. Des. 2012, 44, 155–163. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef]
- Kengla, C.; Kidiyoor, A.; Murphy, S.V. Bioprinting Complex 3D Tissue and Organs. In Kidney Transplantation, Bioengineering, and Regeneration: Kidney Transplantation in the Regenerative Medicine Era; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 957–971. [Google Scholar] [CrossRef]
- Skardal, A. Bioprinting essentials of cell and protein viability. In Essentials of 3D Biofabrication and Translation; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 1–17. [Google Scholar] [CrossRef]
- Salaris, F.A. Rosa, Construction of 3D in vitro models by bioprinting human pluripotent stem cells: Challenges and opportunities. Brain Res. 2019, 1723, 146393. [Google Scholar] [CrossRef]
- Augustine, R.; Kalva, S.; Ahmad, R.; Zahid, A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted cancer models: Revolutionizing personalized cancer therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef]
- Chen, C.; Sereti, K.; Wu, B.; Ardehali, R. Translational aspects of cardiac cell therapy. J. Cell. Mol. Med. 2015, 19, 1757–1772. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.-Y.; Tse, H.-F. Multipotent (adult) and pluripotent stem cells for heart regeneration: What are the pros and cons? Stem Cell Res. Ther. 2013, 4, 151. [Google Scholar] [CrossRef]
- Gálvez-Montón, C.; Prat-Vidal, C.; Roura, S.; Soler-Botija, C.; Bayes-Genis, A. Cardiac Tissue Engineering and the Bioartificial Heart. Rev. Española De Cardiol. (Engl. Ed.) 2013, 66, 391–399. [Google Scholar] [CrossRef]
- Dmitriev, R.; Borisov, S.; Düssmann, H.; Sun, S.; Müller, B.; Prehn, J.; Baklaushev, V.; Klimant, I.; Papkovsky, D. Versatile conjugated polymer nanoparticles for high-resolution O2 imaging in cells and 3D tissue models. ACS Nano 2015, 9, 5275–5288. [Google Scholar] [CrossRef]
- Müller, B.; Zhdanov, A.V.; Borisov, S.; Foley, T.; Okkelman, I.; Tsytsarev, V.; Tang, Q.; Erzurumlu, R.; Chen, Y.; Zhang, H.; et al. Nanoparticle-Based Fluoroionophore for Analysis of Potassium Ion Dynamics in 3D Tissue Models and In Vivo. Adv. Funct. Mater. 2018, 28, 1704598. [Google Scholar] [CrossRef]
- Goliwas, K.; Richter, J.; Pruitt, H.; Araysi, L.; Anderson, N.; Samant, R.; Lobo-Ruppert, S.; Berry, J.; Frost, A. Methods to Evaluate Cell Growth, Viability, and Response to Treatment in a Tissue Engineered Breast Cancer Model. Sci. Rep. 2017, 7, 14167. [Google Scholar] [CrossRef] [Green Version]
- Bardsley, K.; Deegan, A.; El Haj, A.; Yang, Y. Current state-of-the-art 3D tissue models and their compatibility with live cell imaging. Adv. Exp. Med. Biol. 2017, 1035, 3–18. [Google Scholar] [CrossRef]
- Elliott, A. Confocal Microscopy: Principles and Modern Practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef]
- Fischer, E.; Hansen, B.; Nair, V.; Hoyt, F.; Dorward, D. Scanning electron microscopy. Curr. Protoc. Microbiol. 2012, 25, 2B.2.1–2B.2.47. [Google Scholar] [CrossRef]
- Misof, B.; Roschger, P.; Fratzl, P. Imaging mineralized tissues in vertebrates. In Comprehensive Biomaterials; Elsevier: Amsterdam, The Netherlands, 2011; pp. 407–426. [Google Scholar] [CrossRef]
- Ishikawa-Ankerhold, H.; Ankerhold, R.; Drummen, G. Advanced fluorescence microscopy techniques-FRAP, FLIP, FLAP, FRET and FLIM. Molecules 2012, 17, 4047–4132. [Google Scholar] [CrossRef] [Green Version]
- Donius, A.; Bougoin, S.V.; Taboas, J. FRET imaging in three-dimensional hydrogels. J. Vis. Exp. 2016, 2016, 54135. [Google Scholar] [CrossRef]
- Sekar, R.; Periasamy, A. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J. Cell Biol. 2003, 160, 629–633. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, J.; Pitris, C.; Boppart, S.; Brezinski, M. Optical coherence tomography: An emerging technology for biomedical imaging and optical biopsy. Neoplasia 2000, 2, 9–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Xu, M.; Zhang, L.; Zhou, Q.; Luo, L. Automated quantitative assessment of three-dimensional bioprinted hydrogel scaffolds using optical coherence tomography. Biomed. Opt. Express 2016, 7, 894–910. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Larina, I.V. High-resolution imaging techniques in tissue engineering. In Monitoring and Evaluation of Biomaterials and Their Performance In Vivo; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 151–180. [Google Scholar] [CrossRef]
- Meddens, M.; de Keijzer, S.; Cambi, A. High Spatiotemporal Bioimaging Techniques to Study the Plasma Membrane Nanoscale Organization. In Fluorescence Microscopy: Super-Resolution and Other Novel Techniques; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 49–63. [Google Scholar] [CrossRef]
- Lee, S.; Lee, B.; Lee, J.; Kim, S.; Kim, J.; Jeong, Y.; Jin, S. Microscale diffusion measurements and simulation of a scaffold with a permeable strut. Int. J. Mol. Sci. 2013, 14, 20157–20170. [Google Scholar] [CrossRef]
- Zheng, K.; Rupnick, M.; Liu, B.; Brezinski, M. Three Dimensional OCT in the Engineering of Tissue Constructs: A Potentially Powerful Tool for Assessing Optimal Scaffold Structure. Open Tissue Eng. Regen. Med. J. 2009, 2, 8–13. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.; Luo, L.; Zhou, Y.; Si, P. Iterative feedback bio-printing-derived cell-laden hydrogel scaffolds with optimal geometrical fidelity and cellular controllability. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]











| S.No | Characteristics | 2D Cell Cultures | 3D Cell Cultures | Ref |
| 1 | Cell morphology | Cell shape is elongated and grows on a flat 2D surface. | Natural cell shape is preserved with spheroids or organoids structures and with other 3D models | [11] |
| 2 | Cell proliferation | Cell growth in 2 dimensions is rapid and does not mimic in vivo | Cell growth is realistic under 3D culture conditions | [12] |
| 3 | Cell and ECM interactions | Growing on a flat surface is not mimicking the native tissue environment. There is no cell and ECM interactions. | Cells and ECM interact with each other and make a 3D environment such as the existing interactions in native tissues. These models reduce the cost of in vivo testing. | [1,13] |
| 4 | Cell–Cell Interactions | Multi-cell interactions cannot mimic the native organ environment. | Multi-cell interactions in different 3D tissue models can mimic the native environment. | [14] |
| 5 | Cell differentiation | Less resemblance to the native tissue. | Mimics native tissue-like differentiation and markers expression are close to the native tissues. | [15] |
| 6 | Vasculature | 2D co-culture vasculature studies do not mimic the native vascular system. | Ability to incorporate complex vasculature in the 3D model | [16] |
| 7 | Protein and gene expression | Lack of 3D culture conditions, the expression levels may not show much resemblance to in vivo | Show resemblance with in vivo environment | [3] |
| 8 | Drug response efficacy | Low or sometimes not predictable due to cells growing on plastic substrate | Predictable as structure is—in vivo environment. | [3,17] |
| 9 | Apoptosis and viability (tumor models) | Sensitive to study the target drugs | High resistance to the anti-cancer drugs, which replicates the in vivo environment. | [12,18] |
| 10 | Mechanical stimulation | Mechanical stimulation may not mimic the native tissue | We can apply mechanical stimulus according to the native environment and it is an accurate representation of cells in vivo. | [14] |
| 11 | Physiological relevance | Highly non-relevant to the physiological environment. | Feasible to make physiologically relevant nutritional and oxygen conditions. | [1] |
| 12 | Exposure to culture condition | All cells receive nutrients and growth supplements equally. | The core site of the models will not obtain enough nutrients. Different approaches are explored to improve upon the nutrient and oxygen diffusion to the core site. | [13] |
| 13 | Experimentation and analysis | Easy to handle and highly reproducible. | Handling is difficult when compared to 2D cultures, less reproducible, and difficult to handle. | [19] |
| 14 | Characterizations | Easy to characterize the cells for experiment with any instrument. Well-established characterization techniques are available. | Difficult to characterize the 3D models. Specific instrumentation is required. Time consuming and not so well-established. | [20] |
| 15 | Cost | Inexpensive and well established | Expensive and requires further standardization. | [18] |
| Method | SCPL | Electrospinning | 3D Bioprinting |
|---|---|---|---|
| Advantages | Control over pore size and density, ease of fabrication | Highly efficient and well-understood process, in-expensive, controllable fiber dimensions, high resolution | Moderate resolution, diverse choice of biomaterials, dimensional control, cheap, variation in geometry, spatial control of cells deposition. |
| Disadvantages | Residual solvents and salts, generally isotropic properties, weaker mechanical integrity | 3D architecture is challenging, toxic solvents, lower mechanical properties, inhomogeneous cell distribution, time consuming. | Finding the right bio-inks with the right sterilizable and cross-linkable properties. Limited devices can print whole organ, application dependent |
| Characterization | Properties | Method | |
|---|---|---|---|
| Physical | Swelling Ratio | Fraction increase in weight of hydrogel due to water absorption | Weighing difference |
| Degradation rate | Fractional decrease in material to facilitate tissue growth | Collagenase and Weight measurement | |
| Porosity and Morphology | Determination of porous structure to facilitate cellular impregnation and proliferation | Scanning Electron Microscope (SEM), Brunauer, Emmett and Teller (BET) technique | |
| Chemical | FTIR Spectroscopy | Investigate formation of Chemical Bonds | Standard FTIR Protocol |
| H-NMR | Investigate the Molecular Structure | Standard NMR Protocol | |
| Degree of Functionalization | Quantify functional groups | Habeeb Method | |
| Mechanical | Mechanical Loading | Determine elasticity of biomaterials | Youngs Modulus, Tension, Compression, Shear, Torsion, Yield Strength, Ultimate Yield Strength |
| Rheology | Determine viscoelastic characteristics such as Shear Thinning, Viscosity, Storage and Loss Modulus | Rheometer, Viscometer | |
| Printability | Determine optimum parameters to enable efficient printing e.g., Needle Gauge, Print speed, Extrusion Pressure, Geometry, laser power, UV Crosslinking | Based on the tissue architecture equipment | |
| Biological | Cell Volume | Optimize quantity of cells required for functional models | Cell Culture methods |
| Cell Viability and Proliferation | Determine and monitor the response and health of cells, survivability, and spread tissue model | CCK-8, MTT, XTT, Live/Dead | |
| Cytotoxicity, Adhesion | Determine toxicity of biomaterials on cells and how well cells adhere to surface of tissue models. | Fluorescence microscopy, Confocal Microscopy | |
| Immunostaining | Identification and assessment of the topographical distribution of cells, proteins, and detect antigen levels. Eg F-Actin/DAPI | Immunohistochemistry, Flow cytometry, Western Blotting, ELISA, Immuno-electron Microscopy | |
| Cell category | Pros | Cons | Reference |
|---|---|---|---|
| Mesenchymal Stem Cells | Can differentiate into many types of cells | Limited in quantity, differentiation capacity diminishes with age | [113,114] |
| Adult stem cell | Can differentiate into cells of the lineage it belongs to, main function is to repair the organ they are found in | Limited in quantity, expensive Limited capacity to divide | [85] |
| Adipose Derived Stem Cells | Multipotent, easily isolated, easily available | Low survival | [115] |
| Embryonic stem cells | Can differentiate into any cells in the right conditions, | Ethical concerns, allogenic and hence require immunosuppressants | [114] |
| Induced Pluripotent Stem Cells | Can be reprogrammed to embryonic stem cell-like state, can differentiate into any cell, | teratomas formation | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shyam, R.; Reddy, L.V.K.; Palaniappan, A. Fabrication and Characterization Techniques of In Vitro 3D Tissue Models. Int. J. Mol. Sci. 2023, 24, 1912. https://doi.org/10.3390/ijms24031912
Shyam R, Reddy LVK, Palaniappan A. Fabrication and Characterization Techniques of In Vitro 3D Tissue Models. International Journal of Molecular Sciences. 2023; 24(3):1912. https://doi.org/10.3390/ijms24031912
Chicago/Turabian StyleShyam, Rohin, L. Vinod Kumar Reddy, and Arunkumar Palaniappan. 2023. "Fabrication and Characterization Techniques of In Vitro 3D Tissue Models" International Journal of Molecular Sciences 24, no. 3: 1912. https://doi.org/10.3390/ijms24031912
APA StyleShyam, R., Reddy, L. V. K., & Palaniappan, A. (2023). Fabrication and Characterization Techniques of In Vitro 3D Tissue Models. International Journal of Molecular Sciences, 24(3), 1912. https://doi.org/10.3390/ijms24031912